This is “Applied Nuclear Chemistry”, section 20.5 from the book Principles of General Chemistry (v. 1.0M). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
20.5 Applied Nuclear Chemistry
Learning Objective
- To understand how nuclear reactors function.
The ever-increasing energy needs of modern societies have led scientists and engineers to develop ways of harnessing the energy released by nuclear reactions. To date, all practical applications of nuclear power have been based on nuclear fission reactions. Although nuclear fusion offers many advantages in principle, technical difficulties in achieving the high energies required to initiate nuclear fusion reactions have thus far precluded using fusion for the controlled release of energy. In this section, we describe the various types of nuclear power plants that currently generate electricity from nuclear reactions, along with some possible ways to harness fusion energy in the future. In addition, we discuss some of the applications of nuclear radiation and radioisotopes, which have innumerable uses in medicine, biology, chemistry, and industry.

Pitchblende. Pitchblende, the major uranium ore, consisting mainly of uranium oxide.
Nuclear Reactors
When a critical mass of a fissile isotope is achieved, the resulting flux of neutrons can lead to a self-sustaining reaction. A variety of techniques can be used to control the flow of neutrons from such a reaction, which allows nuclear fission reactions to be maintained at safe levels. Many levels of control are required, along with a fail-safe design, because otherwise the chain reaction can accelerate so rapidly that it releases enough heat to melt or vaporize the fuel and the container, a situation that can release enough radiation to contaminate the surrounding area. Uncontrolled nuclear fission reactions are relatively rare, but they have occurred at least 18 times in the past. The most recent event resulted from the damaged Fukushima Dai-ichi plant after the March 11, 2011, earthquake and tsunami that devastated Japan. The plant used fresh water for cooling nuclear fuel rods to maintain controlled, sustainable nuclear fission. When the water supply was disrupted, so much heat was generated that a partial meltdown occurred. Radioactive iodine levels in contaminated seawater from the plant were over 4300 times the regulated safety limit. To put this in perspective, drinking one liter of fresh water with this level of contamination is the equivalent to receiving double the annual dose of radiation that is typical for a person. Dismantling the plant and decontaminating the site is estimated to require 30 years at a cost of approximately $12 billion.
There is compelling evidence that uncontrolled nuclear chain reactions occurred naturally in the early history of our planet, about 1.7 billion years ago in uranium deposits near Oklo in Gabon, West Africa (Figure 20.19 "A “Fossil Nuclear Reactor” in a Uranium Mine Near Oklo in Gabon, West Africa"). The natural abundance of 235U 2 billion years ago was about 3%, compared with 0.72% today; in contrast, the “fossil nuclear reactor” deposits in Gabon now contain only 0.44% 235U. An unusual combination of geologic phenomena in this region apparently resulted in the formation of deposits of essentially pure uranium oxide containing 3% 235U, which coincidentally is identical to the fuel used in many modern nuclear plants. When rainwater or groundwater saturated one of these deposits, the water acted as a natural moderator that decreased the kinetic energy of the neutrons emitted by radioactive decay of 235U, allowing the neutrons to initiate a chain reaction. As a result, the entire deposit “went critical” and became an uncontrolled nuclear chain reaction, which is estimated to have produced about 100 kW of power. It is thought that these natural nuclear reactors operated only intermittently, however, because the heat released would have vaporized the water. Removing the water would have shut down the reactor until the rocks cooled enough to allow water to reenter the deposit, at which point the chain reaction would begin again. This on–off cycle is believed to have been repeated for more than 100,000 years, until so much 235U was consumed that the deposits could no longer support a chain reaction.
Figure 20.19 A “Fossil Nuclear Reactor” in a Uranium Mine Near Oklo in Gabon, West Africa

More than a billion years ago, a number of uranium-rich deposits in West Africa apparently “went critical,” initiating uncontrolled nuclear fission reactions that may have continued intermittently for more than 100,000 years, until the concentration of uranium-235 became too low to support a chain reaction. This photo shows a geologist standing in a mine dug to extract the concentrated uranium ore. Commercial interest waned rapidly after it was recognized that the uranium ore was severely depleted in uranium-235, the isotope of interest.
In addition to the incident in Japan, another recent instance of an uncontrolled nuclear chain reaction occurred on April 25–26, 1986, at the Chernobyl nuclear power plant in the former Union of Soviet Socialist Republics (USSR; now in the Ukraine; Figure 20.20 "The Chernobyl Nuclear Power Plant"). During testing of the reactor’s turbine generator, a series of mechanical and operational failures caused a chain reaction that quickly went out of control, destroying the reactor core and igniting a fire that destroyed much of the facility and released a large amount of radioactivity. Thirty people were killed immediately, and the high levels of radiation in a 20 mi radius forced nearly 350,000 people to be resettled or evacuated. In addition, the accident caused a disruption to the Soviet economy that is estimated to have cost almost $13 billion. It is somewhat surprising, however, that the long-term health effects on the 600,000 people affected by the accident appear to be much less severe than originally anticipated. Initially, it was predicted that the accident would result in tens of thousands of premature deaths, but an exhaustive study almost 20 yr after the event suggests that 4000 people will die prematurely from radiation exposure due to the accident. Although significant, in fact it represents only about a 3% increase in the cancer rate among the 600,000 people most affected, of whom about a quarter would be expected to eventually die of cancer even if the accident had not occurred.
Figure 20.20 The Chernobyl Nuclear Power Plant

In 1986, mechanical and operational failures during testing at the Chernobyl power plant in the USSR (now in the Ukraine) caused an uncontrolled nuclear chain reaction. The resulting fire destroyed much of the facility and severely damaged the core of the reactor, resulting in the release of large amounts of radiation that was spread over the surrounding area by the prevailing winds. The effects were devastating to the health of the population in the region and to the Soviet economy.
If, on the other hand, the neutron flow in a reactor is carefully regulated so that only enough heat is released to boil water, then the resulting steam can be used to produce electricity. Thus a nuclear reactor is similar in many respects to the conventional power plants discussed in Chapter 5 "Energy Changes in Chemical Reactions", which burn coal or natural gas to generate electricity; the only difference is the source of the heat that converts water to steam.
Light-Water Reactors
We begin our description of nuclear power plants with light-water reactors, which are used extensively to produce electricity in countries such as Japan, Israel, South Korea, Taiwan, and France—countries that lack large reserves of fossil fuels. The essential components of a light-water reactor are depicted in Figure 20.21 "A Light-Water Nuclear Fission Reactor for the Production of Electric Power". All existing nuclear power plants have similar components, although different designs use different fuels and operating conditions. Fuel rods containing a fissile isotope in a structurally stabilized form (such as uranium oxide pellets encased in a corrosion-resistant zirconium alloy) are suspended in a cooling bath that transfers the heat generated by the fission reaction to a secondary cooling system. The heat is used to generate steam for the production of electricity. In addition, control rods are used to absorb neutrons and thereby control the rate of the nuclear chain reaction. Control rods are made of a substance that efficiently absorbs neutrons, such as boron, cadmium, or, in nuclear submarines, hafnium. Pulling the control rods out increases the neutron flux, allowing the reactor to generate more heat, whereas inserting the rods completely stops the reaction, a process called “scramming the reactor.”
Figure 20.21 A Light-Water Nuclear Fission Reactor for the Production of Electric Power
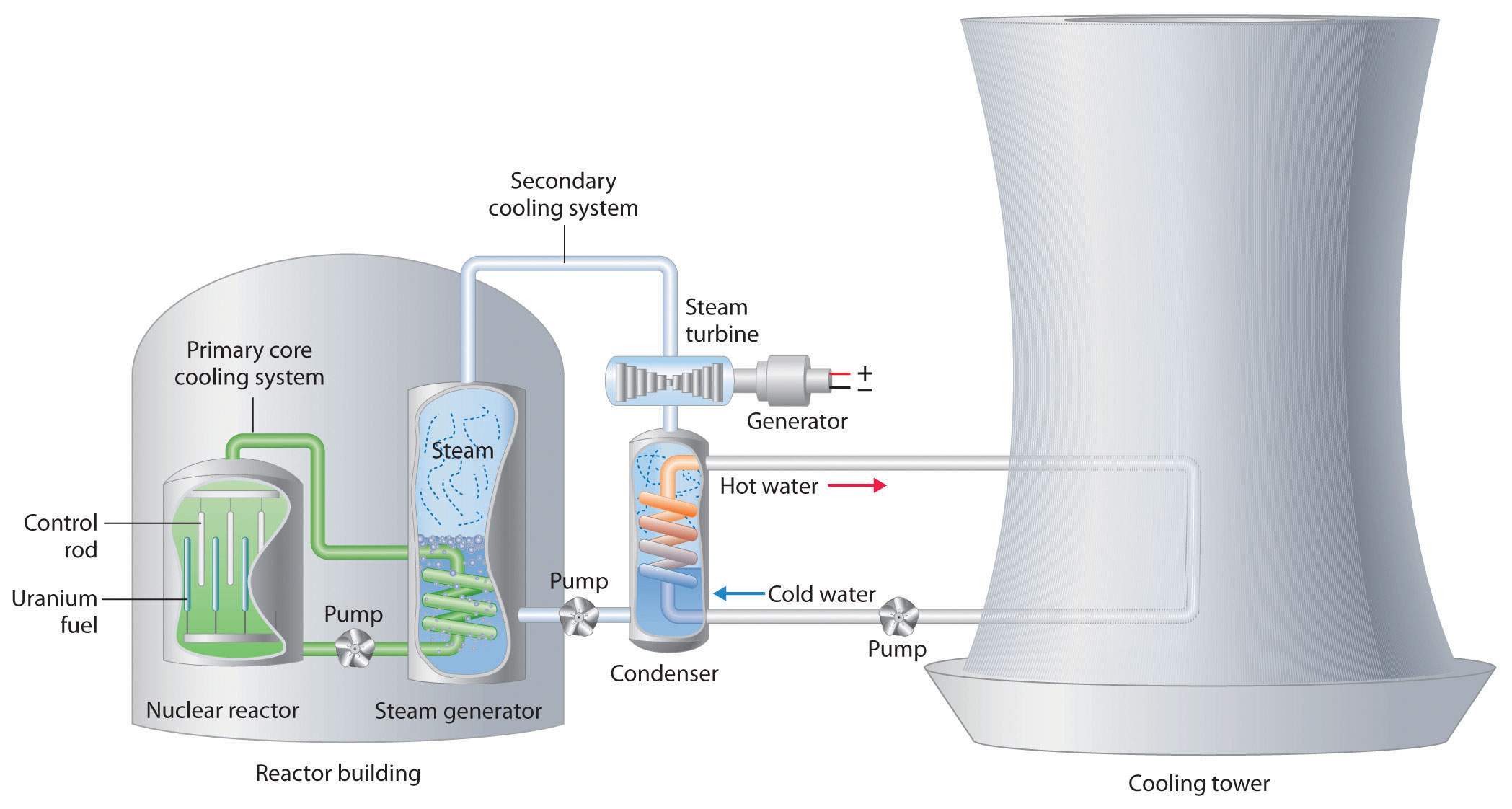
The fuel rods are made of a corrosion-resistant alloy that encases the partially enriched uranium fuel; controlled fission of 235U in the fuel produces heat. Water surrounds the fuel rods and moderates the kinetic energy of the neutrons, slowing them to increase the probability that they will induce fission. Control rods that contain elements such as boron, cadmium, or hafnium, which are very effective at absorbing neutrons, are used to control the rate of the fission reaction. A heat exchanger is used to boil water in a secondary cooling system, creating steam to drive the turbine and produce electricity. The large hyperbolic cooling tower, which is the most visible portion of the facility, condenses the steam in the secondary cooling circuit; it is often located at some distance from the actual reactor.
Despite this apparent simplicity, many technical hurdles must be overcome for nuclear power to be an efficient and safe source of energy. Uranium contains only 0.72% uranium-235, which is the only naturally occurring fissile isotope of uranium. Because this abundance is not enough to support a chain reaction, the uranium fuel must be at least partially enriched in 235U, to a concentration of about 3%, for it to be able to sustain a chain reaction. At this level of enrichment, a nuclear explosion is impossible; far higher levels of enrichment (greater than or equal to 90%) are required for military applications such as nuclear weapons or the nuclear reactors in submarines. Enrichment is accomplished by converting uranium oxide to UF6, which is volatile and contains discrete UF6 molecules. Because 235UF6 and 238UF6 have different masses, they have different rates of effusion and diffusion, and they can be separated using a gas diffusion process, as described in Chapter 10 "Gases". Another difficulty is that neutrons produced by nuclear fission are too energetic to be absorbed by neighboring nuclei, and they escape from the material without inducing fission in nearby 235U nuclei. Consequently, a moderator must be used to slow the neutrons enough to allow them to be captured by other 235U nuclei. High-speed neutrons are scattered by substances such as water or graphite, which decreases their kinetic energy and increases the probability that they will react with another 235U nucleus. The moderator in a light-water reactor is the water that is used as the primary coolant. The system is highly pressurized to about 100 atm to keep the water from boiling at 100°C.
All nuclear reactors require a powerful cooling system to absorb the heat generated in the reactor core and create steam that is used to drive a turbine that generates electricity. In 1979, an accident occurred when the main water pumps used for cooling at the nuclear power plant at Three Mile Island in Pennsylvania stopped running, which prevented the steam generators from removing heat. Eventually, the zirconium casing of the fuel rods ruptured, resulting in a meltdown of about half of the reactor core. Although there was no loss of life and only a small release of radioactivity, the accident produced sweeping changes in nuclear power plant operations. The US Nuclear Regulatory Commission tightened its oversight to improve safety.
The main disadvantage of nuclear fission reactors is that the spent fuel, which contains too little of the fissile isotope for power generation, is much more radioactive than the unused fuel due to the presence of many daughter nuclei with shorter half-lives than 235U. The decay of these daughter isotopes generates so much heat that the spent fuel rods must be stored in water for as long as 5 yr before they can be handled. Even then, the radiation levels are so high that the rods must be stored for many, many more years to allow the daughter isotopes to decay to nonhazardous levels. How to store these spent fuel rods for hundreds of years is a pressing issue that has not yet been successfully resolved. As a result, some people are convinced that nuclear power is not a viable option for providing our future energy needs, although a number of other countries continue to rely on nuclear reactors for a large fraction of their energy.
Heavy-Water Reactors
Deuterium (2H) absorbs neutrons much less effectively than does hydrogen (1H), but it is about twice as effective at slowing neutrons. Consequently, a nuclear reactor that uses D2O instead of H2O as the moderator is so efficient that it can use unenriched uranium as fuel. Using a lower grade of uranium reduces operating costs and eliminates the need for plants that produce enriched uranium. Because of the expense of D2O, however, only countries like Canada, which has abundant supplies of hydroelectric power for generating D2O by electrolysis, have made a major investment in heavy-water reactors. (For more information on electrolysis, see Chapter 19 "Electrochemistry".)
Breeder Reactors
A breeder reactor is a nuclear fission reactor that produces more fissionable fuel than it consumes. This does not violate the first law of thermodynamics because the fuel produced is not the same as the fuel consumed. Under heavy neutron bombardment, the nonfissile 238U isotope is converted to 239Pu, which can undergo fission:
Equation 20.40
The overall reaction is thus the conversion of nonfissile 238U to fissile 239Pu, which can be chemically isolated and used to fuel a new reactor. An analogous series of reactions converts nonfissile 232Th to 233U, which can also be used as a fuel for a nuclear reactor. Typically, about 8–10 yr are required for a breeder reactor to produce twice as much fissile material as it consumes, which is enough to fuel a replacement for the original reactor plus a new reactor. The products of the fission of 239Pu, however, have substantially longer half-lives than the fission products formed in light-water reactors.
Nuclear Fusion Reactors
Although nuclear fusion reactions, such as those in Equation 20.38 and Equation 20.39, are thermodynamically spontaneous, the positive charge on both nuclei results in a large electrostatic energy barrier to the reaction. (As you learned in Chapter 18 "Chemical Thermodynamics", thermodynamic spontaneity is unrelated to the reaction rate.) Extraordinarily high temperatures (about 1.0 × 108°C) are required to overcome electrostatic repulsions and initiate a fusion reaction. Even the most feasible such reaction, deuterium–tritium fusion (D–T fusion; Equation 20.39), requires a temperature of about 4.0 × 107°C. Achieving these temperatures and controlling the materials to be fused are extraordinarily difficult problems, as is extracting the energy released by the fusion reaction, because a commercial fusion reactor would require such high temperatures to be maintained for long periods of time. Several different technologies are currently being explored, including the use of intense magnetic fields to contain ions in the form of a dense, high-energy plasma at a temperature high enough to sustain fusion (part (a) in Figure 20.22 "Two Possible Designs for a Nuclear Fusion Reactor"). Another concept employs focused laser beams to heat and compress fuel pellets in controlled miniature fusion explosions (part (b) in Figure 20.22 "Two Possible Designs for a Nuclear Fusion Reactor").
Figure 20.22 Two Possible Designs for a Nuclear Fusion Reactor
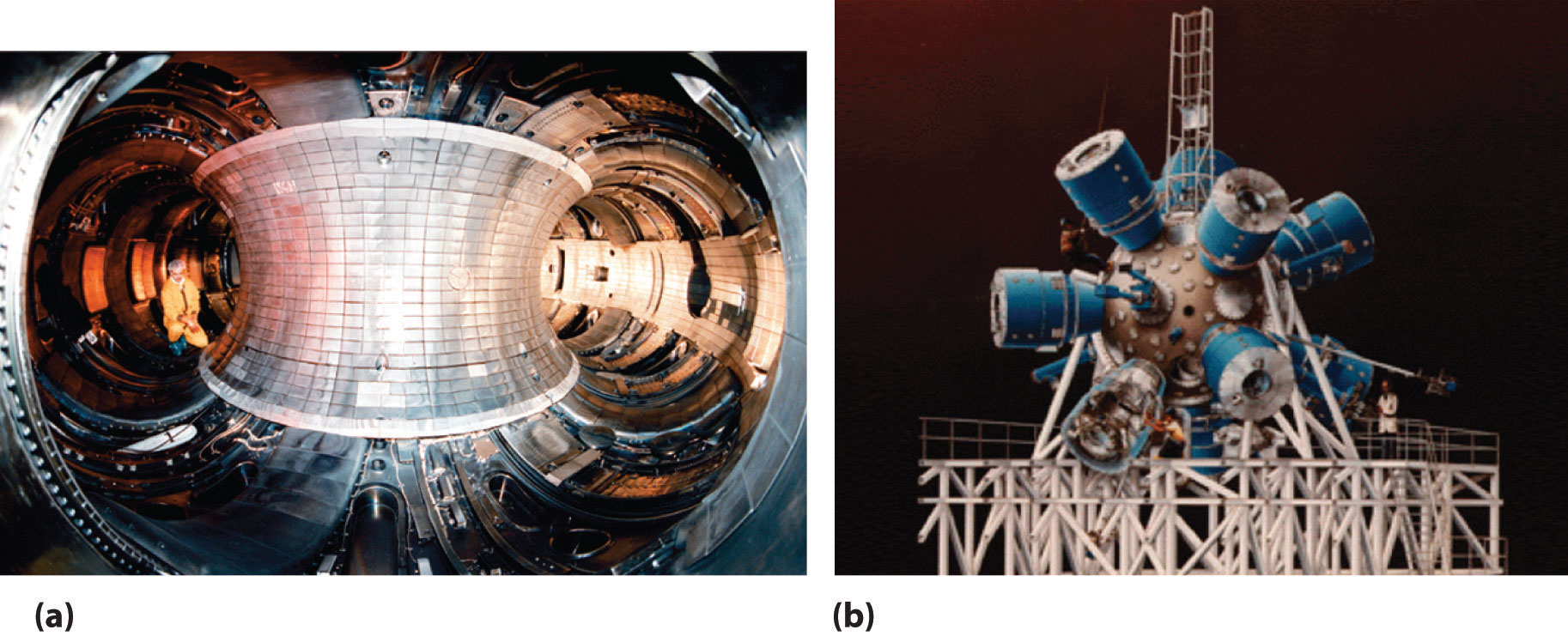
The extraordinarily high temperatures needed to initiate a nuclear fusion reaction would immediately destroy a container made of any known material. (a) One way to avoid contact with the container walls is to use a high-energy plasma as the fuel. Because plasma is essentially a gas composed of ionized particles, it can be confined using a strong magnetic field shaped like a torus (a hollow donut). (b) Another approach to nuclear fusion is inertial confinement, which uses an icosahedral array of powerful lasers to heat and compress a tiny fuel pellet (a mixture of solid LiD and LiT) to induce fusion.
Nuclear reactions such as these are called thermonuclear reactionsA nuclear reaction that requires a great deal of thermal energy to initiate the reaction. because a great deal of thermal energy must be invested to initiate the reaction. The amount of energy released by the reaction, however, is several orders of magnitude greater than the energy needed to initiate it. In principle, a nuclear fusion reaction should thus result in a significant net production of energy. In addition, Earth’s oceans contain an essentially inexhaustible supply of both deuterium and tritium, which suggests that nuclear fusion could provide a limitless supply of energy. Unfortunately, however, the technical requirements for a successful nuclear fusion reaction are so great that net power generation by controlled fusion has yet to be achieved.
The Uses of Radioisotopes
Nuclear radiation can damage biological molecules, thereby disrupting normal functions such as cell division (Table 20.4 "The Effects of a Single Radiation Dose on a 70 kg Human"). Because radiation is particularly destructive to rapidly dividing cells such as tumor cells and bacteria, it has been used medically to treat cancer since 1904, when radium-226 was first used to treat a cancerous tumor. Many radioisotopes are now available for medical use, and each has specific advantages for certain applications.
In modern radiation therapy, radiation is often delivered by a source planted inside the body. For example, tiny capsules containing an isotope such as 192Ir, coated with a thin layer of chemically inert platinum, are inserted into the middle of a tumor that cannot be removed surgically. The capsules are removed when the treatment is over. In some cases, physicians take advantage of the body’s own chemistry to deliver a radioisotope to the desired location. For example, the thyroid glands in the lower front of the neck are the only organs in the body that use iodine. Consequently, radioactive iodine is taken up almost exclusively by the thyroid (part (a) in Figure 20.23 "Medical Imaging and Treatment with Radioisotopes"). Thus when radioactive isotopes of iodine (125I or 131I) are injected into the blood of a patient suffering from thyroid cancer, the thyroid glands filter the radioisotope from the blood and concentrate it in the tissue to be destroyed. In cases where a tumor is surgically inaccessible (e.g., when it is located deep in the brain), an external radiation source such as a 60Co “gun” is used to aim a tightly focused beam of γ rays at it. Unfortunately, radiation therapy damages healthy tissue in addition to the target tumor and results in severe side effects, such as nausea, hair loss, and a weakened immune system. Although radiation therapy is generally not a pleasant experience, in many cases it is the only choice.
Figure 20.23 Medical Imaging and Treatment with Radioisotopes
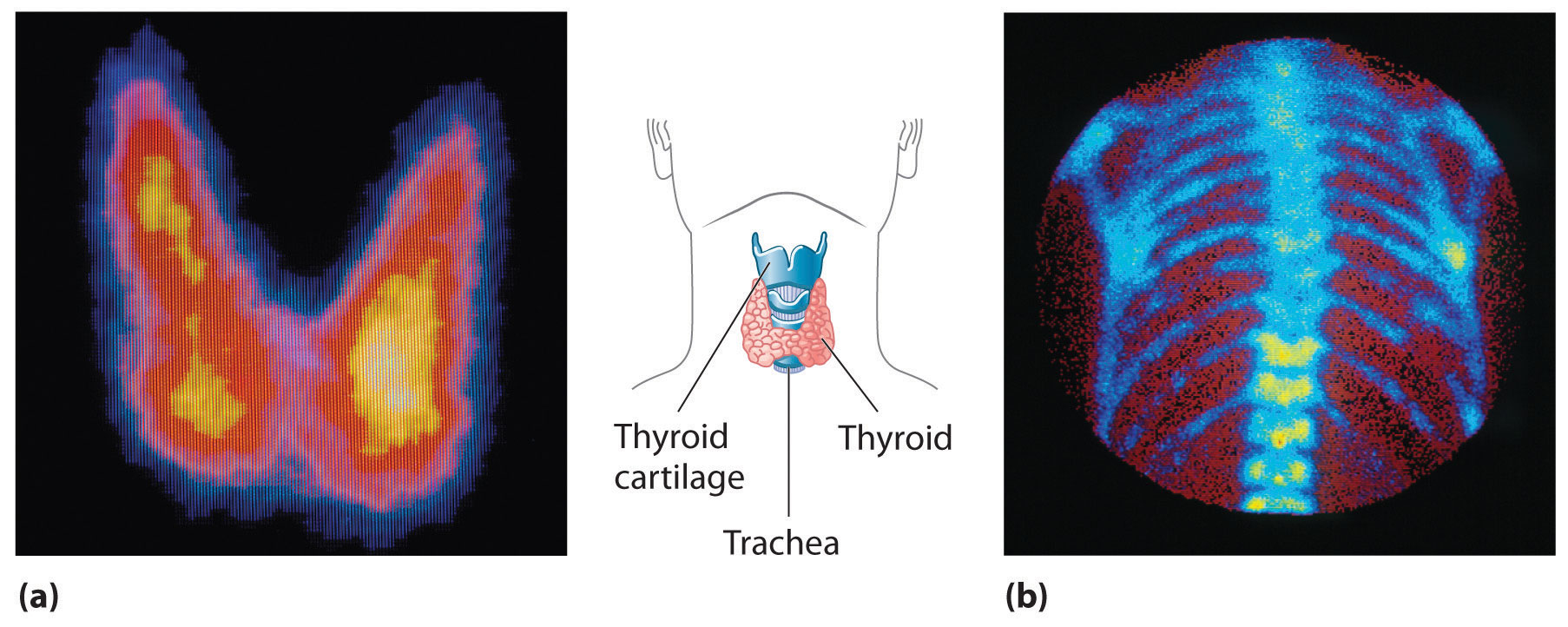
(a) Radioactive iodine is used both to obtain images of the thyroid and to treat thyroid cancer. Injected iodine-123 or iodine-131 is selectively taken up by the thyroid gland, where it is incorporated into the thyroid hormone: thyroxine. Because iodine-131 emits low-energy β particles that are absorbed by the surrounding tissue, it can be used to destroy malignant tissue in the thyroid. In contrast, iodine-123 emits higher-energy γ rays that penetrate tissues readily, enabling it to image the thyroid gland, as shown here. (b) Some technetium compounds are selectively absorbed by cancerous cells within bones. The yellow spots show that a primary cancer has metastasized (spread) to the patient’s spine (lower center) and ribs (right center).
A second major medical use of radioisotopes is medical imaging, in which a radioisotope is temporarily localized in a particular tissue or organ, where its emissions provide a “map” of the tissue or the organ. Medical imaging uses radioisotopes that cause little or no tissue damage but are easily detected. One of the most important radioisotopes for medical imaging is 99mTc. Depending on the particular chemical form in which it is administered, technetium tends to localize in bones and soft tissues, such as the heart or the kidneys, which are almost invisible in conventional x-rays (part (b) in Figure 20.23 "Medical Imaging and Treatment with Radioisotopes"). Some properties of other radioisotopes used for medical imaging are listed in Table 20.5 "Radioisotopes Used in Medical Imaging and Treatment".
Table 20.5 Radioisotopes Used in Medical Imaging and Treatment
| Isotope | Half-Life | Tissue |
|---|---|---|
| 18F | 110 min | brain |
| 24Na | 15 h | circulatory system |
| 32P | 14 days | eyes, liver, and tumors |
| 59Fe | 45 days | blood and spleen |
| 60Co | 5.3 yr | external radiotherapy |
| 99mTc | 6 h | heart, thyroid, liver, kidney, lungs, and skeleton |
| 125I | 59.4 days | thyroid, prostate, and brain |
| 131I | 8 days | thyroid |
| 133Xe | 5 days | lungs |
| 201Tl | 3 days | heart |
Because γ rays produced by isotopes such as 131I and 99mTc are emitted randomly in all directions, it is impossible to achieve high levels of resolution in images that use such isotopes. However, remarkably detailed three-dimensional images can be obtained using an imaging technique called positron emission tomography (PET). The technique uses radioisotopes that decay by positron emission, and the resulting positron is annihilated when it collides with an electron in the surrounding matter. (For more information on positron emission, see Section 20.2 "Nuclear Reactions".) In the annihilation process, both particles are converted to energy in the form of two γ rays that are emitted simultaneously and at 180° to each other:
Equation 20.41
With PET, biological molecules that have been “tagged” with a positron-emitting isotope such as 18F or 11C can be used to probe the functions of organs such as the brain.
Another major health-related use of ionizing radiation is the irradiation of food, an effective way to kill bacteria such as Salmonella in chicken and eggs and potentially lethal strains of Escherichia coli in beef. Collectively, such organisms cause almost 3 million cases of food poisoning annually in the United States, resulting in hundreds of deaths. Figure 20.24 "The Preservation of Strawberries with Ionizing Radiation" shows how irradiation dramatically extends the storage life of foods such as strawberries. Although US health authorities have given only limited approval of this technique, the growing number of illnesses caused by antibiotic-resistant bacteria is increasing the pressure to expand the scope of food irradiation.
Figure 20.24 The Preservation of Strawberries with Ionizing Radiation

Fruits such as strawberries can be irradiated by high-energy γ rays to kill bacteria and prolong their storage life. The nonirradiated strawberries on the left are completely spoiled after 15 days in storage, but the irradiated strawberries on the right show no visible signs of spoilage under the same conditions.
One of the more unusual effects of radioisotopes is in dentistry. Because dental enamels contain a mineral called feldspar (KAlSi3O8, which is also found in granite rocks), teeth contain a small amount of naturally occurring radioactive 40K. The radiation caused by the decay of 40K results in the emission of light (fluorescence), which gives the highly desired “pearly white” appearance associated with healthy teeth.
In a sign of how important nuclear medicine has become in diagnosing and treating illnesses, the medical community has become alarmed at the global shortage of 99Tc, a radioisotope used in more than 30 million procedures a year worldwide. Two reactors that produce 60% of the world’s radioactive 99Mo, which decays to 99Tc, are operating beyond their intended life expectancies. Moreover, because most of the reactors producing 99Mo use weapons-grade uranium (235U), which decays to 99Mo during fission, governments are working to phase out civilian uses of technology to produce 99Mo because of concerns that the technology can be used to produce nuclear weapons. Engineers are currently focused on how to make key medical isotopes with other alternatives that don’t require fission. One promising option is by removing a neutron from 100Mo, a stable isotope that makes up about 10% of natural molybdenum, transmuting it to 99Mo.
In addition to the medical uses of radioisotopes, radioisotopes have literally hundreds of other uses. For example, smoke detectors contain a tiny amount of 241Am, which ionizes the air in the detector so an electric current can flow through it. Smoke particles reduce the number of ionized particles and decrease the electric current, which triggers an alarm. Another application is the “go-devil” used to detect leaks in long pipelines. It is a packaged radiation detector that is inserted into a pipeline and propelled through the pipe by the flowing liquid. Sources of 60Co are attached to the pipe at regular intervals; as the detector travels along the pipeline, it sends a radio signal each time it passes a source. When a massive leak causes the go-devil to stop, the repair crews know immediately which section of the pipeline is damaged. Finally, radioactive substances are used in gauges that measure and control the thickness of sheets and films. As shown in Figure 20.25 "Using Radiation to Control the Thickness of a Material", thickness gauges rely on the absorption of either β particles (by paper, plastic, and very thin metal foils) or γ rays (for thicker metal sheets); the amount of radiation absorbed can be measured accurately and is directly proportional to the thickness of the material.
Figure 20.25 Using Radiation to Control the Thickness of a Material
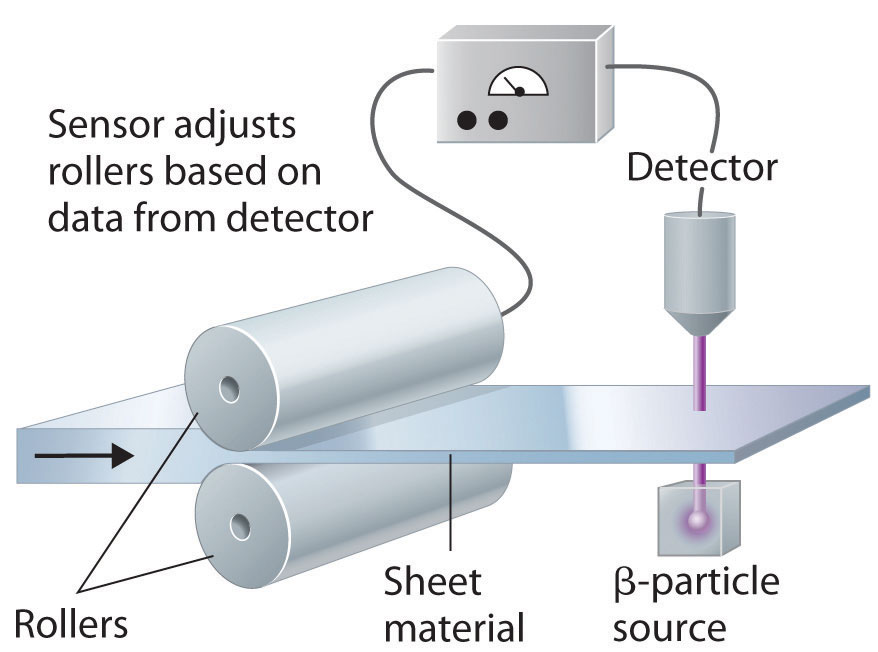
Because the amount of radiation absorbed by a material is proportional to its thickness, radiation can be used to control the thickness of plastic film, tin foil, or paper. As shown, a beta emitter is placed on one side of the material being produced and a detector on the other. An increase in the amount of radiation that reaches the detector indicates a decrease in the thickness of the material and vice versa. The output of the detector can thus be used to control the thickness of the material.
Summary
In nuclear power plants, nuclear reactions generate electricity. Light-water reactors use enriched uranium as a fuel. They include fuel rods, a moderator, control rods, and a powerful cooling system to absorb the heat generated in the reactor core. Heavy-water reactors use unenriched uranium as a fuel because they use D2O as the moderator, which scatters and slows neutrons much more effectively than H2O. A breeder reactor produces more fissionable fuel than it consumes. A nuclear fusion reactor requires very high temperatures. Fusion reactions are thermonuclear reactions because they require high temperatures for initiation. Radioisotopes are used in both radiation therapy and medical imaging.
Key Takeaway
- All practical applications of nuclear power have been based on nuclear fission reactions, which nuclear power plants use to generate electricity.
Conceptual Problems
-
In nuclear reactors, two different but interrelated factors must be controlled to prevent a mishap that could cause the release of unwanted radiation. How are these factors controlled?
-
What are the three principal components of a nuclear reactor? What is the function of each component?
-
What is meant by the term enrichment with regard to uranium for fission reactors? Why does the fuel in a conventional nuclear reactor have to be “enriched”?
-
The plot in a recent spy/action movie involved the threat of introducing stolen “weapons-grade” uranium, which consists of 93.3% 235U, into a fission reactor that normally uses a fuel containing about 3% 235U. Explain why this could be catastrophic.
-
Compare a heavy-water reactor with a light-water reactor. Why are heavy-water reactors less widely used? How do these two reactor designs compare with a breeder reactor?
-
Conventional light-water fission reactors require enriched fuel. An alternative reactor is the so-called heavy-water reactor. The components of the two different reactors are the same except that instead of using water (H2O), the moderator in a heavy-water reactor is D2O, known as “heavy water.” Because D2O is more efficient than H2O at slowing neutrons, heavy-water reactors do not require fuel enrichment to support fission. Why is D2O more effective at slowing neutrons, and why does this allow unenriched fuels to be used?
-
Isotopes emit γ rays in random directions. What difficulties do these emissions present for medical imaging? How are these difficulties overcome?
-
If you needed to measure the thickness of 1.0 mm plastic sheets, what type of radiation would you use? Would the radiation source be the same if you were measuring steel of a similar thickness? What is your rationale? Would you want an isotope with a long or short half-life for this device?
Answers
-
Neutron flow is regulated by using control rods that absorb neutrons, whereas the speed of the neutrons produced by fission is controlled by using a moderator that slows the neutrons enough to allow them to react with nearby fissile nuclei.
-
-
-
-
-
-
It is difficult to pinpoint the exact location of the nucleus that decayed. In contrast, the collision of a positron with an electron causes both particles to be annihilated, and in the process, two gamma rays are emitted in opposite directions, which makes it possible to identify precisely where a positron emitter is located and to create detailed images of tissues.
-
Numerical Problems
-
Palladium-103, which decays via electron capture and emits x-rays with an energy of 3.97 × 10−2 MeV, is often used to treat prostate cancer. Small pellets of the radioactive metal are embedded in the prostate gland. This provides a localized source of radiation to a very small area, even though the tissue absorbs only about 1% of the x-rays. Due to its short half-life, all of the palladium will decay to a stable isotope in less than a year. If a doctor embeds 50 pellets containing 2.50 mg of 103Pd in the prostate gland of a 73.9 kg patient, what is the patient’s radiation exposure over the course of a year?
-
Several medical treatments use cobalt-60m, which is formed by bombarding cobalt with neutrons to produce a highly radioactive gamma emitter that undergoes 4.23 × 1016 emissions/(s·kg) of pure cobalt-60. The energy of the gamma emission is 5.86 × 10−2 MeV. Write the balanced nuclear equation for the formation of this isotope. Is this a transmutation reaction? If a 55.3 kg patient received a 0.50 s exposure to a 0.30 kg cobalt-60 source, what would the exposure be in rads? Predict the potential side effects of this dose.




