This is “Amino Acids, Proteins, and Enzymes”, chapter 18 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
Chapter 18 Amino Acids, Proteins, and Enzymes
Opening Essay
The 1923 Nobel Prize in Medicine or Physiology was awarded to Frederick Grant Banting and John James Richard Macleod for their discovery of the protein insulin. In 1958, the Nobel Prize in Chemistry was awarded to Frederick Sanger for his discoveries concerning the structure of proteins and, in particular, the structure of insulin. What is so important about insulin that two Nobel Prizes have been awarded for work on this protein?
Insulin is a hormone that is synthesized in the pancreas. (For more information about hormones, see Chapter 17 "Lipids", Section 17.4 "Steroids".) Insulin stimulates the transport of glucose into cells throughout the body and the storage of glucose as glycogen. People with diabetes do not produce insulin or use it properly. The isolation of insulin in 1921 led to the first effective treatment for these individuals.
Figure 18.1 An Insulin Pump
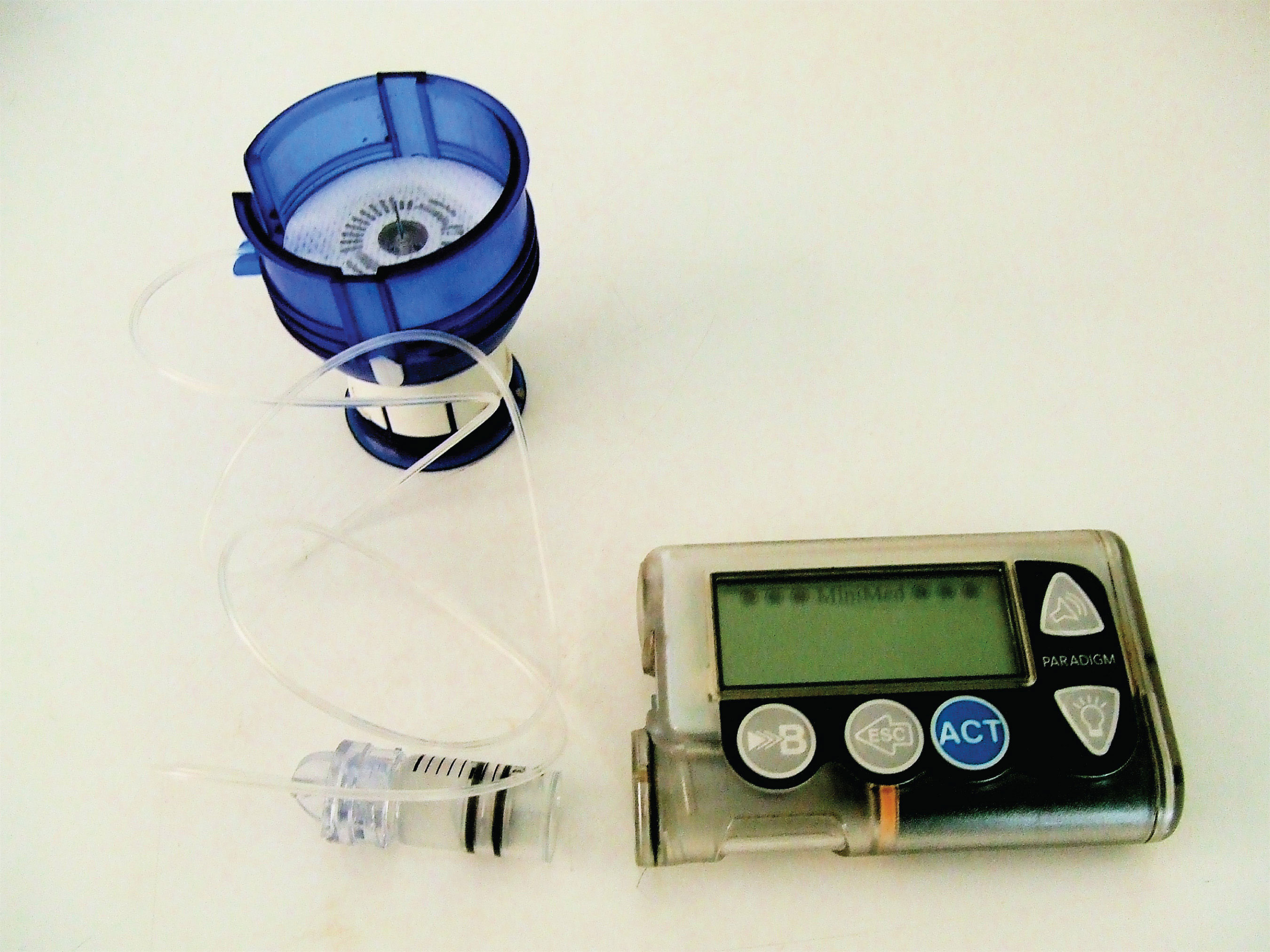
ProteinsA compound of high molar mass consisting largely or entirely of amino acids linked together. may be defined as compounds of high molar mass consisting largely or entirely of chains of amino acids. Their masses range from several thousand to several million daltons (Da). In addition to carbon, hydrogen, and oxygen atoms, all proteins contain nitrogen and sulfur atoms, and many also contain phosphorus atoms and traces of other elements. Proteins serve a variety of roles in living organisms and are often classified by these biological roles, which are summarized in Table 18.1 "Classification of Proteins by Biological Function". Muscle tissue is largely protein, as are skin and hair. Proteins are present in the blood, in the brain, and even in tooth enamel. Each type of cell in our bodies makes its own specialized proteins, as well as proteins common to all or most cells.
Note
The dalton is a unit of mass used by biochemists and biologists. It is equivalent to the atomic mass unit. A 30,000 Da protein has a molar mass of 30,000 u.
Table 18.1 Classification of Proteins by Biological Function
| Classification | Biological Function | Example |
|---|---|---|
| enzymes | accelerate biological reactions | α-Amylase catalyzes the hydrolysis of starch and glycogen. |
| structural | provide strength and structure | Keratin is the primary protein of hair and wool. |
| contractile | muscle contraction; cell division | Myosin is one protein needed for the contraction of muscles. |
| transport | transport substances from one place to another | Hemoglobin transports oxygen from the lungs throughout the body. |
| regulatory | regulate the functioning of other proteins | Insulin regulates the activity of specific enzymes in the body. |
| storage | provide storage of essential nutrients | Ovalbumin stores amino acids in the egg white that will be used by the developing bird. |
| protection | protect cells or the organism from foreign substances | Immunoglobulins recognize and breakdown foreign molecules. |
We begin our study of proteins by looking at the properties and reactions of amino acids, which is followed by a discussion of how amino acids link covalently to form peptides and proteins. We end the chapter with a discussion of enzymes—the proteins that act as catalysts in the body.




