This is “Carbohydrates”, section 16.1 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
16.1 Carbohydrates
Learning Objective
- Recognize carbohydrates and classify them as mono-, di-, or polysaccharides.
All carbohydratesA compound composed of carbon, hydrogen, and oxygen atoms that is a polyhydroxy aldehyde or ketone or a compound that can be broken down to form such a compound. It is one of the three main components of the human diet. consist of carbon, hydrogen, and oxygen atoms and are polyhydroxy aldehydes or ketones or are compounds that can be broken down to form such compounds. Examples of carbohydrates include starch, fiber, the sweet-tasting compounds called sugars, and structural materials such as cellulose. The term carbohydrate had its origin in a misinterpretation of the molecular formulas of many of these substances. For example, because its formula is C6H12O6, glucose was once thought to be a “carbon hydrate” with the structure C6·6H2O.
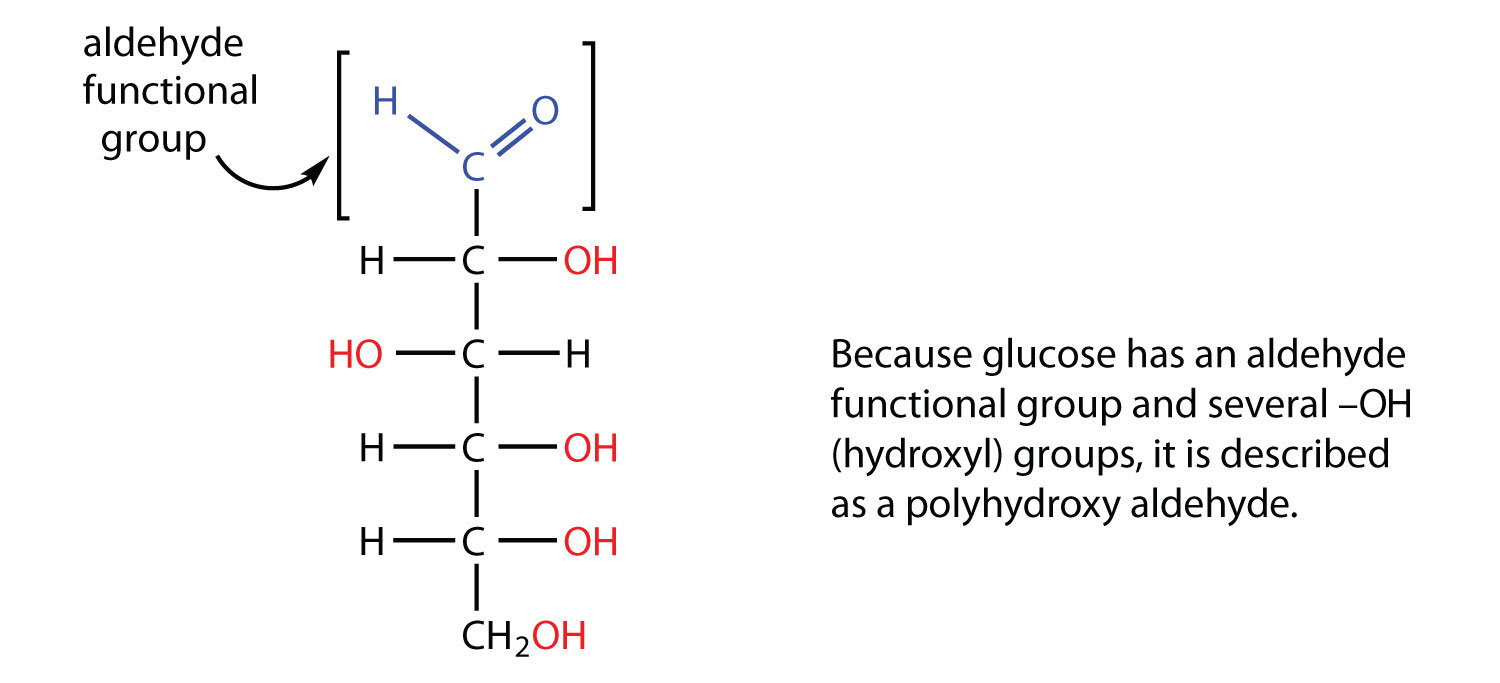
Example 1
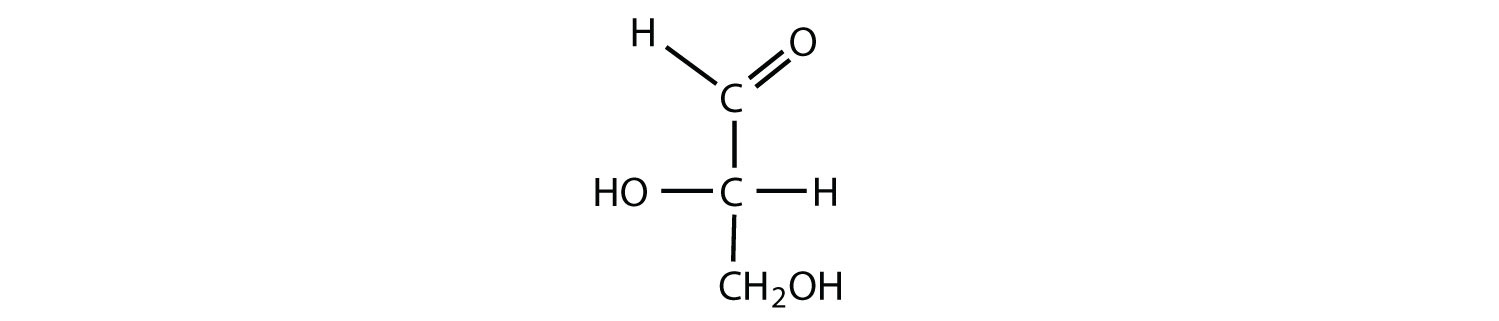
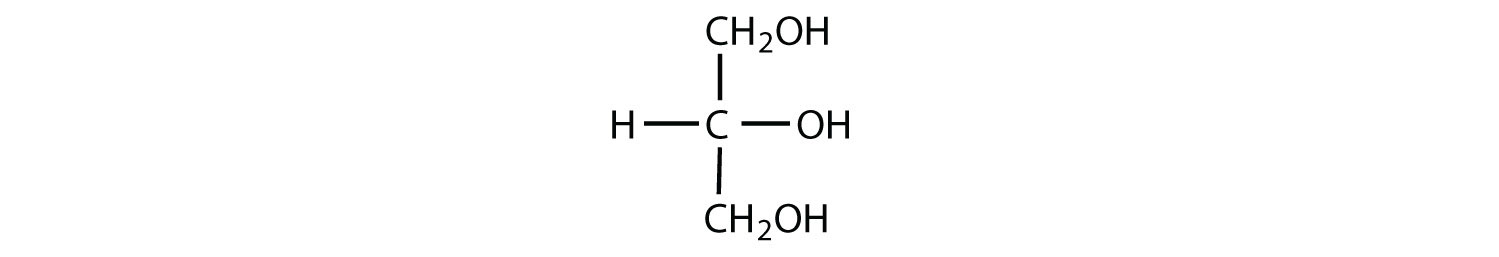
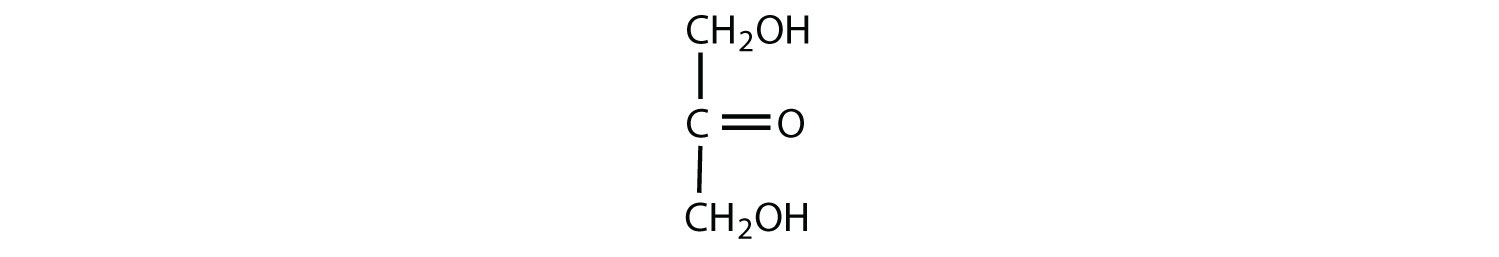
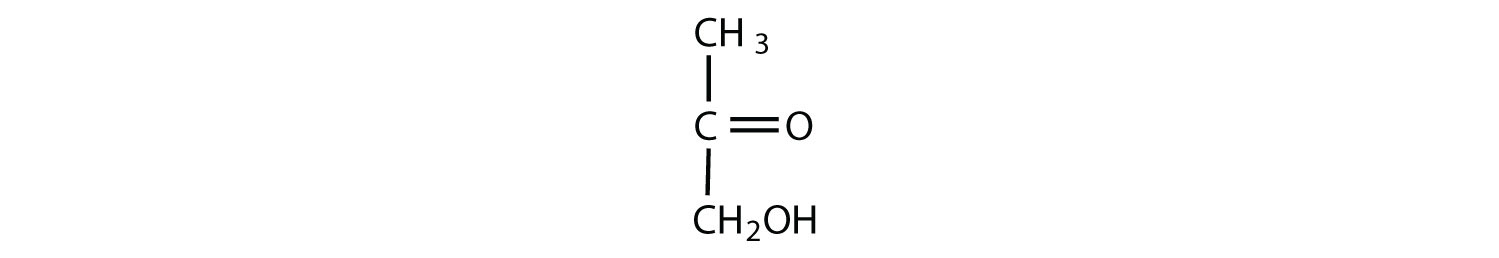
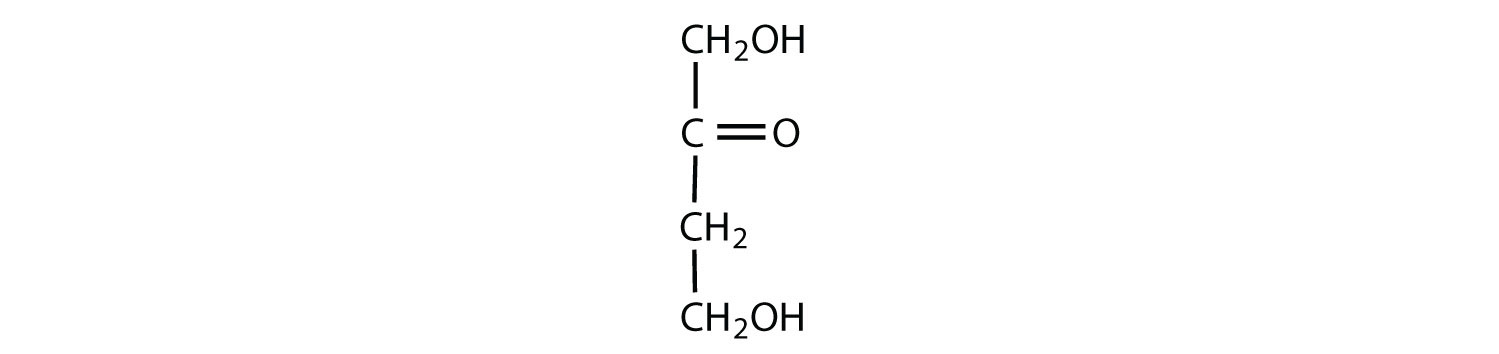
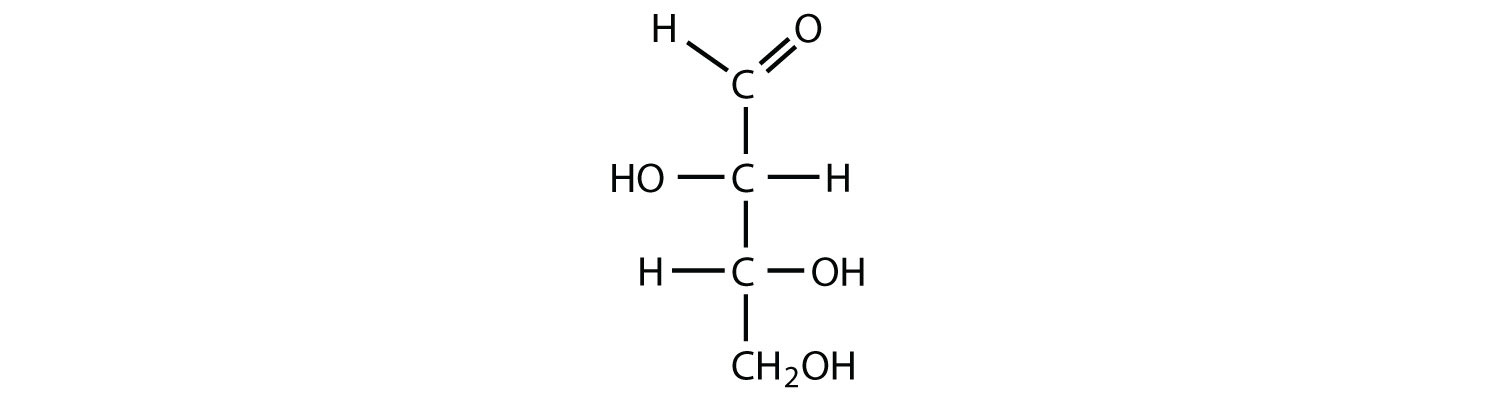
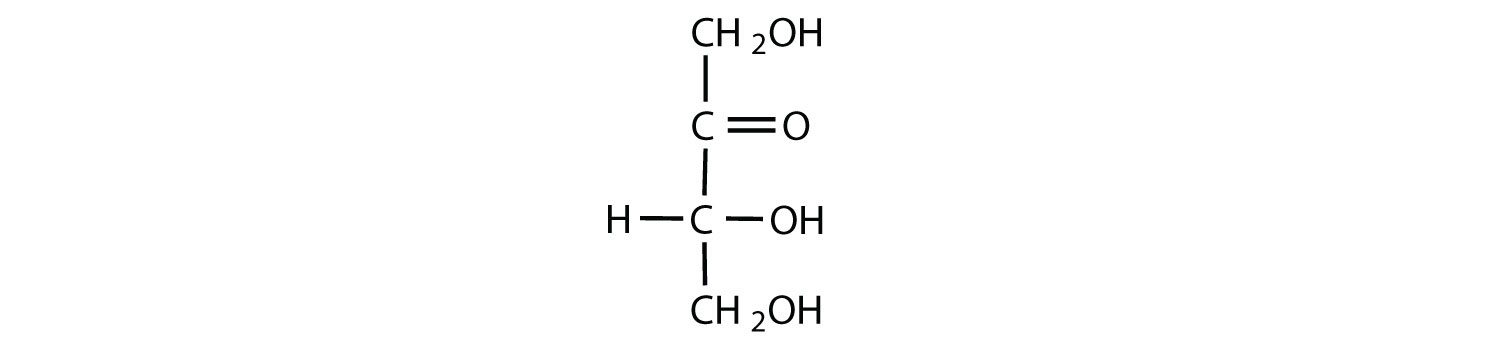
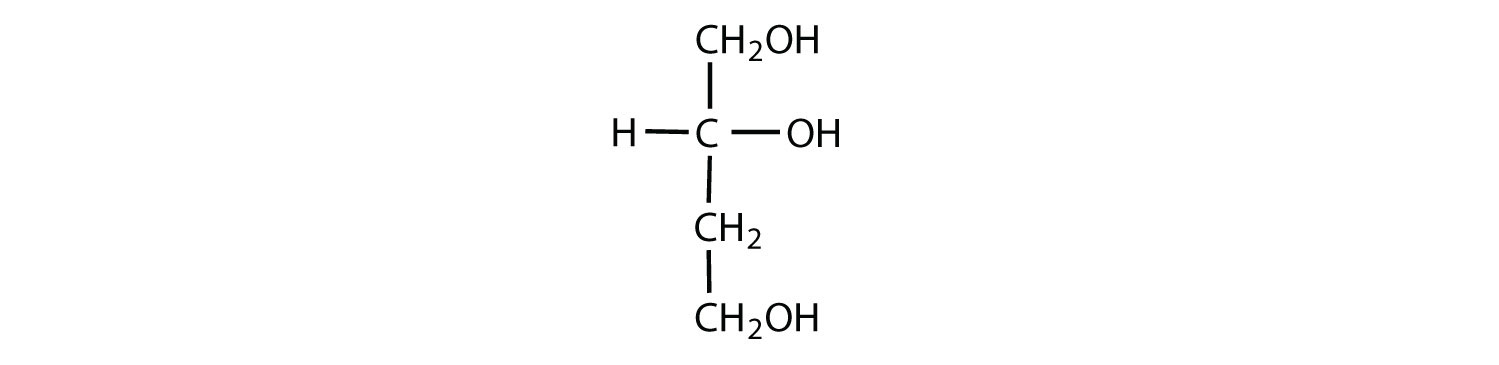
Which compounds would be classified as carbohydrates?
Solution
- This is a carbohydrate because the molecule contains an aldehyde functional group with OH groups on the other two carbon atoms.
- This is not a carbohydrate because the molecule does not contain an aldehyde or a ketone functional group.
- This is a carbohydrate because the molecule contains a ketone functional group with OH groups on the other two carbon atoms.
- This is not a carbohydrate; although it has a ketone functional group, one of the other carbons atoms does not have an OH group attached.
Skill-Building Exercise
Which compounds would be classified as carbohydrates?
Green plants are capable of synthesizing glucose (C6H12O6) from carbon dioxide (CO2) and water (H2O) by using solar energy in the process known as photosynthesisThe process by which plants use solar energy to convert carbon dioxide and water to glucose.:
6CO2 + 6H2O + 686 kcal → C6H12O6 + 6O2(The 686 kcal come from solar energy.) Plants can use the glucose for energy or convert it to larger carbohydrates, such as starch or cellulose. Starch provides energy for later use, perhaps as nourishment for a plant’s seeds, while cellulose is the structural material of plants. We can gather and eat the parts of a plant that store energy—seeds, roots, tubers, and fruits—and use some of that energy ourselves. Carbohydrates are also needed for the synthesis of nucleic acids and many proteins and lipids.
Animals, including humans, cannot synthesize carbohydrates from carbon dioxide and water and are therefore dependent on the plant kingdom to provide these vital compounds. We use carbohydrates not only for food (about 60%–65% by mass of the average diet) but also for clothing (cotton, linen, rayon), shelter (wood), fuel (wood), and paper (wood).
The simplest carbohydrates—those that cannot be hydrolyzed to produce even smaller carbohydrates—are called monosaccharidesThe simplest carbohydrate that cannot be hydrolyzed to produce smaller carbohydrate molecules.. Two or more monosaccharides can link together to form chains that contain from two to several hundred or thousand monosaccharide units. Prefixes are used to indicate the number of such units in the chains. DisaccharideA carbohydrate containing two monosaccharide units. molecules have two monosaccharide units, trisaccharide molecules have three units, and so on. Chains with many monosaccharide units joined together are called polysaccharidesA carbohydrate containing many monosaccharide units.. All these so-called higher saccharides can be hydrolyzed back to their constituent monosaccharides.
Note
Compounds that cannot be hydrolyzed will not react with water to form two or more smaller compounds.
Concept Review Exercises
-
Why is photosynthesis important?
-
Identify the differences among monosaccharides, disaccharides, and polysaccharides.
Answers
-
Photosynthesis is the process by which solar energy is used to reduce carbon dioxide to carbohydrates, which are needed for energy by plants and other living organisms that eat plants.
-
A monosaccharide is the simplest carbohydrate and cannot be hydrolyzed to produce a smaller carbohydrate; a disaccharide is composed of two monosaccharide units; and a polysaccharide contains many saccharide units.
Key Takeaways
- Carbohydrates are an important group of biological molecules that includes sugars and starches.
- Photosynthesis is the process by which plants use energy from sunlight to synthesize carbohydrates.
- A monosaccharide is the simplest carbohydrate and cannot be hydrolyzed to produce a smaller carbohydrate molecule. Disaccharides contain two monosaccharide units, and polysaccharides contain many monosaccharide units.
Exercises
-
When an aqueous solution of trehalose is heated, two molecules of glucose are produced for each molecule of trehalose. Is trehalose a monosaccharide, a disaccharide, or a polysaccharide?
-
When an aqueous solution of arabinose is heated, no other molecules are produced. Is arabinose a monosaccharide, a disaccharide, or a polysaccharide?
Answer
-
Trehalose is a disaccharide because it is hydrolyzed into two molecules of glucose (a monosaccharide).
-