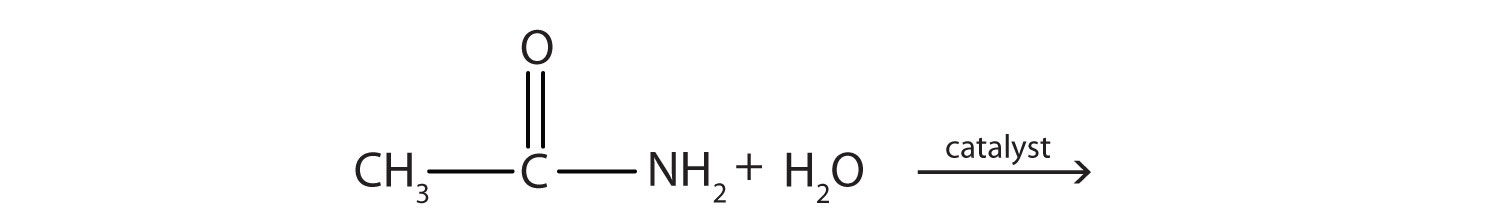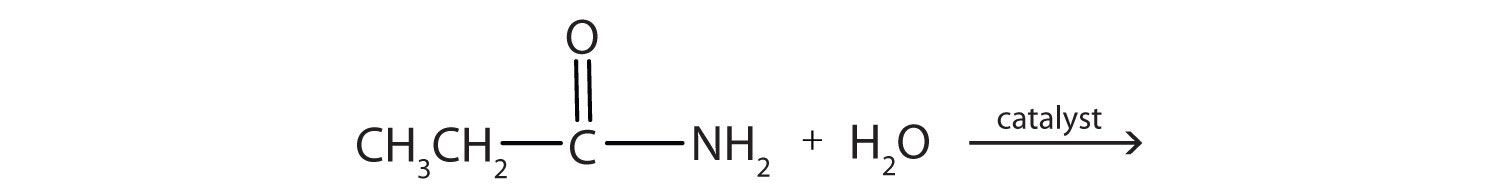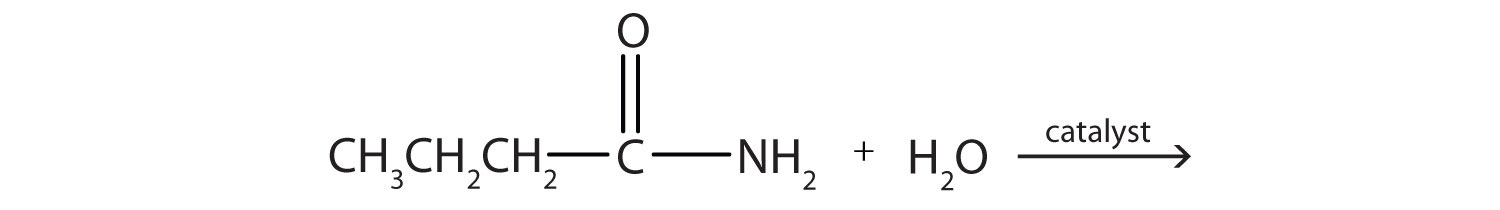This is “Chemical Properties of Amides: Hydrolysis”, section 15.17 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
15.17 Chemical Properties of Amides: Hydrolysis
Learning Objective
- Identify the typical reaction that amides undergo.
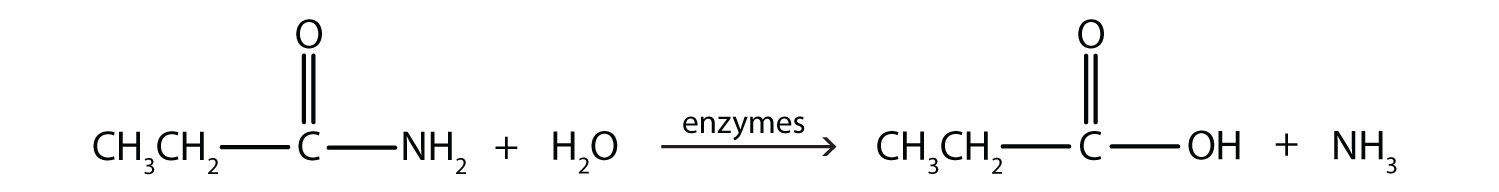
Generally, amides resist hydrolysis in plain water, even after prolonged heating. In the presence of added acid or base, however, hydrolysis proceeds at a moderate rate. In living cells, amide hydrolysis is catalyzed by enzymes. Amide hydrolysis is illustrated in the following example:
Note
Hydrolysis of an amide in acid solution actually gives a carboxylic acid and the salt of ammonia or an amine (the ammonia or amine initially formed is neutralized by the acid). Basic hydrolysis gives a salt of the carboxylic acid and ammonia or an amine.
Example 17
Write the equation for the hydrolysis of each compound.
- butyramide
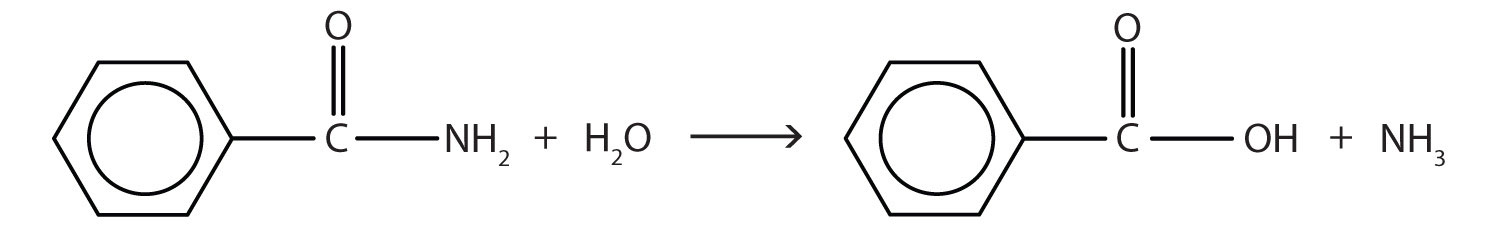
- benzamide
Solution
-
The hydrolysis of a simple amide produces an organic acid and ammonia. Butyramide thus yields butyric acid and ammonia.

-
The hydrolysis of an amide produces an organic acid and ammonia. Benzamide thus yields benzoic acid and ammonia.
Skill-Building Exercise
-
propionamide (propanamide)
-
hexanamide
Write the equation for the hydrolysis of each compound.
Career Focus: Athletic Trainer
Athletic training is an allied health-care profession recognized by the American Medical Association. The athletic trainer’s role is to recognize, evaluate, and provide immediate care for athletic injuries; prevent athletic injuries by taping, bandaging, and bracing vulnerable body parts; make referrals to medical doctors when necessary; and rehabilitate injured athletes. Athletic trainers work in high schools, colleges, and other organizations where athletics programs are found. Athletic trainers usually have a degree from an accredited athletic training program whose curriculum includes such basic science courses as biology, chemistry, and physics. These studies provide the necessary background for more applied courses, such as anatomy and physiology, exercise physiology, kinesiology, and nutrition. Knowledge of chemistry is necessary for understanding pharmacological and medical terminology. For example, athletic trainers must understand the action of numerous drugs, many of which are esters, amines, or amides like those mentioned in this chapter.
Athletic trainers may have administrative duties, such as the responsibility for ordering supplies. They also need to be able to evaluate nutritional supplements because providing the wrong one can get an athlete banned from competition and may bring sanctions against a school. In short, the athletic trainer is responsible for the overall health and well-being of the athletes in his or her charge.
Concept Review Exercises
-
What are the products of the hydrolysis of an amide?
-
When the amide CH3CH2CH2CH2CONH2 is hydrolyzed in an NaOH solution, the products are CH3CH2CH2CH2COO−Na+ and NH3. What products are obtained when CH3CH2CH2CH2CONH2 is hydrolyzed in an hydrochloric acid solution?
Answers
-
a carboxylic acid and ammonia or an amine
-
CH3CH2CH2CH2COOH and NH4Cl
Key Takeaway
- The hydrolysis of an amide produces a carboxylic acid and ammonia or an amine.
Exercises
-
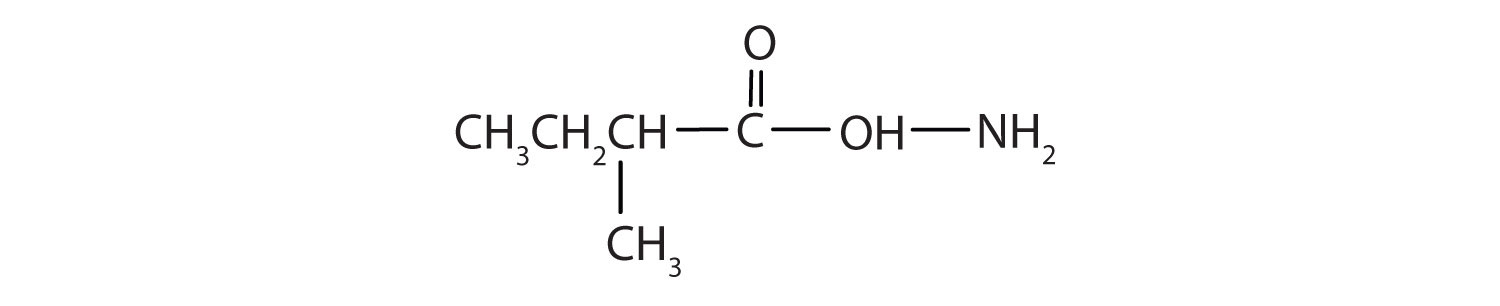
Complete each equation.
-
-
Complete each equation.
-
Answer
-
- CH3COOH + NH3
-
-