This is “Reactions That Form Alcohols”, section 14.4 from the book Introduction to Chemistry: General, Organic, and Biological (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
14.4 Reactions That Form Alcohols
Learning Objective
- Describe how to prepare alcohols from alkenes.
Methanol is prepared by combining hydrogen gas and carbon monoxide at high temperatures and pressures in the presence of a catalyst composed of zinc oxide (ZnO) and chromium oxide (Cr2O3) catalyst:
Methanol is an important solvent and is used as an automotive fuel, either as the pure liquid—as in some racing cars—or as an additive in gasoline.
Note
Nearly 2 billion gallons of methanol are produced each year in the United States by the catalytic reduction of carbon monoxide with hydrogen gas.
Many simple alcohols are made by the hydration of alkenes. (For more information about the hydration of alkenes, see Chapter 13 "Unsaturated and Aromatic Hydrocarbons", Section 13.4 "Chemical Properties of Alkenes".) Ethanol is made by the hydration of ethylene in the presence of a catalyst such as sulfuric acid (H2SO4).
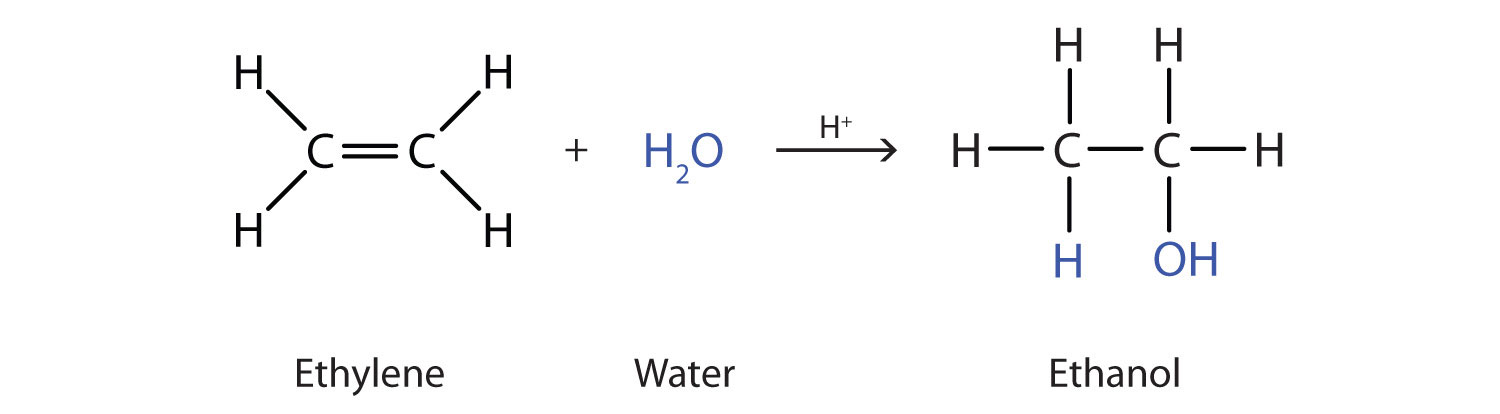
In a similar manner, isopropyl alcohol is produced by the addition of water to propene (propylene).

Note
Additional Exercise 19 describes how to use a generalization called Markovnikov’s rule to predict the results when the addition of water to an alcohol has two possible products.
Example 3
Write the equation for the reaction of 2-butene with water to form 2-butanol. Indicate that sulfuric acid is used as a catalyst.
Solution
First write the condensed structural formula of 2-butene and indicate that it reacts with water. Then write the condensed structural formula of 2-butanol after the reaction arrow to indicate that it is the product. Finally, write the formula for the catalyst above the arrow.
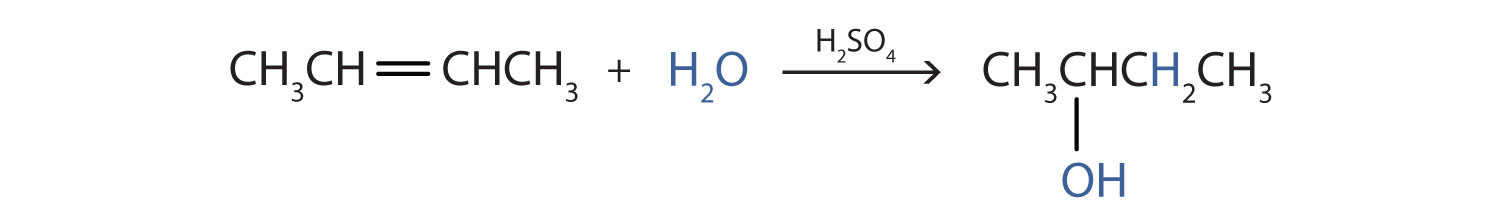
Skill-Building Exercise
-
Write the equation for the reaction of cyclopentene with water to form cyclopentanol. Indicate that phosphoric acid (H3PO4) is used as a catalyst.
Note
Many OH compounds in living systems are formed by alkene hydration. Here is an example that occurs in the Krebs cycle: fumarate is hydrated to form malate. (For more information about the Krebs cycle, see Chapter 20 "Energy Metabolism", Section 20.4 "Stage III of Catabolism".)
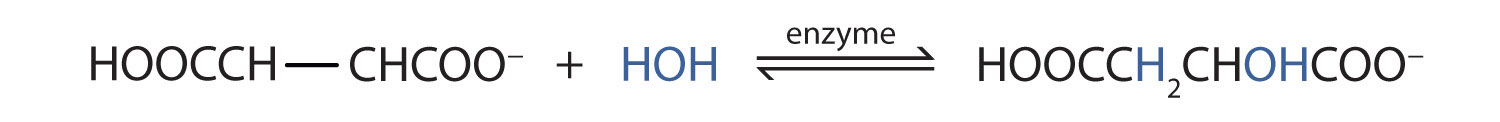
In addition to its preparation from ethylene, ethanol is made by the fermentation of sugars or starch from various sources (potatoes, corn, wheat, rice, etc.). Fermentation is catalyzed by enzymes found in yeast and proceeds by an elaborate multistep mechanism. We can represent the overall process as follows:
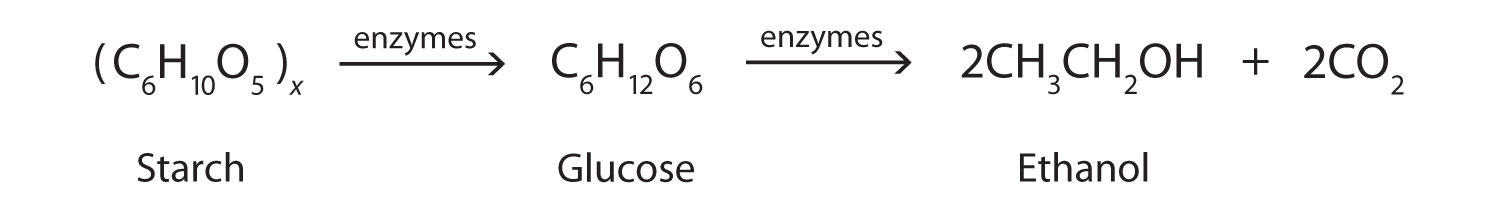
To Your Health: The Physiological Effects of Alcohols
Methanol is quite poisonous to humans. Ingestion of as little as 15 mL of methanol can cause blindness, and 30 mL (1 oz) can cause death. However, the usual fatal dose is 100 to 150 mL. The main reason for methanol’s toxicity is that we have liver enzymes that catalyze its oxidation to formaldehyde, the simplest member of the aldehyde family (Section 14.9 "Aldehydes and Ketones: Structure and Names"):
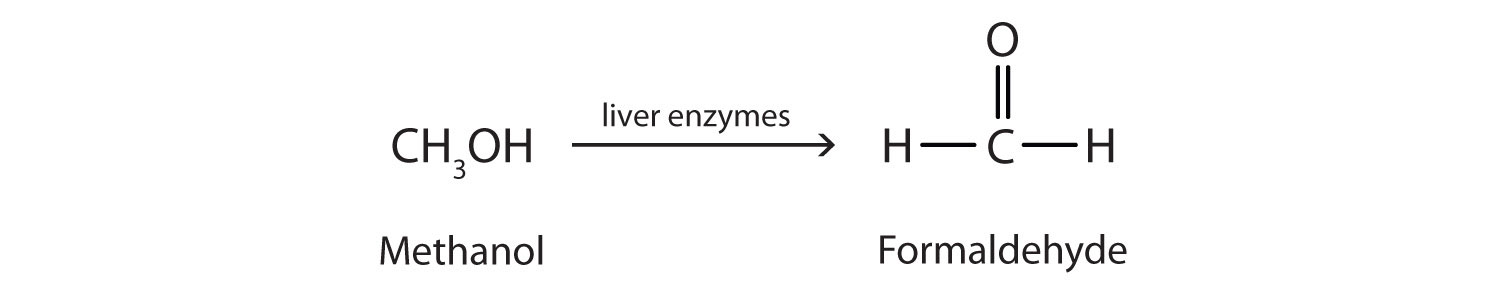
Formaldehyde reacts rapidly with the components of cells, coagulating proteins in much the same way that cooking coagulates an egg. This property of formaldehyde accounts for much of the toxicity of methanol.
Note
Organic and biochemical equations are frequently written showing only the organic reactants and products. In this way, we focus attention on the organic starting material and product, rather than on balancing complicated equations.
Ethanol is oxidized in the liver to acetaldehyde:
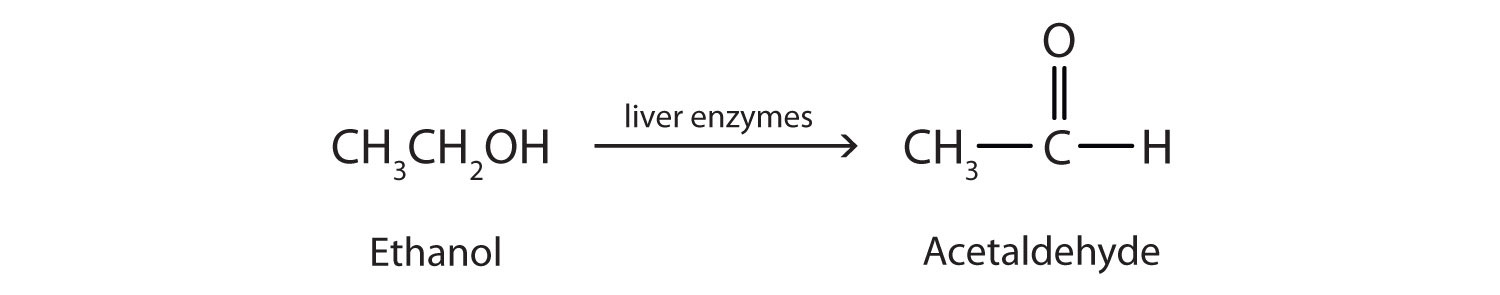
The acetaldehyde is in turn oxidized to acetic acid (HC2H3O2), a normal constituent of cells, which is then oxidized to carbon dioxide and water. Even so, ethanol is potentially toxic to humans. The rapid ingestion of 1 pt (about 500 mL) of pure ethanol would kill most people, and acute ethanol poisoning kills several hundred people each year—often those engaged in some sort of drinking contest. Ethanol freely crosses into the brain, where it depresses the respiratory control center, resulting in failure of the respiratory muscles in the lungs and hence suffocation. Ethanol is believed to act on nerve cell membranes, causing a diminution in speech, thought, cognition, and judgment.
Rubbing alcohol is usually a 70% aqueous solution of isopropyl alcohol. It has a high vapor pressure, and its rapid evaporation from the skin produces a cooling effect. It is toxic when ingested but, compared to methanol, is less readily absorbed through the skin.
Concept Review Exercises
-
Why is methanol more toxic than ethanol?
-
How does rubbing alcohol cool a feverish patient?
Answers
-
Methanol is oxidized to formaldehyde, which destroys tissue; ethanol is oxidized to acetaldehyde and then acetic acid, a normal metabolite.
-
Evaporation removes heat.
Key Takeaways
- Many alcohols are made by the hydration of alkenes.
- Ethanol can be made by the fermentation of sugars or starch from various sources.
Exercises
-
From what alkene is ethanol made? Draw its condensed structural formula.
-
Can methanol be made from an alkene? Explain.
Answer
-
ethylene; CH2=CH2
-




