This is “Alkyl Halides and Alcohols”, section 16.3 from the book Beginning Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
16.3 Alkyl Halides and Alcohols
Learning Objectives
- Define functional group.
- Identify and name a simple alkyl halide.
- Identify and name a simple alcohol.
- Predict the product(s) of an elimination reaction of an alkyl halide or an alcohol.
A functional groupA collection of atoms or bonds with certain characteristic reactions. is any collection of atoms and/or bonds with certain characteristic chemical reactions. We have already seen two functional groups: the C–C double bond and the C–C triple bond. They undergo certain characteristic chemical reactions—for example, the addition of a halogen across the multiple bond.
The presence of a halogen atom (F, Cl, Br, or I; also, X is used to represent any halogen atom) is one of the simplest functional groups. Organic compounds that contain a halogen atom are called alkyl halidesAn organic compound that contains a halogen atom.. We have already seen some examples of alkyl halides when the addition of halogens across double and triple bonds was introduced in Chapter 16 "Organic Chemistry", Section 16.2 "Branched Hydrocarbons"; the products of these reactions were alkyl halides.
A simple alkyl halide can be named like an ionic salt, first by stating the name of the parent alkane as a substituent group (with the -yl suffix) and then the name of the halogen as if it were the anion. So CH3Cl has the common name of methyl chloride, while CH3CH2Br is ethyl bromide and CH3CH2CH2I is propyl iodide. However, this system is not ideal for more complicated alkyl halides.
The systematic way of naming alkyl halides is to name the halogen as a substituent, just like an alkyl group, and use numbers to indicate the position of the halogen atom on the main chain. The name of the halogen as a substituent comes from the stem of the element’s name plus the ending -o, so the substituent names are fluoro-, chloro-, bromo- and iodo-. If there is more than one of a certain halogen, we use numerical prefixes to indicate the number of each kind, just as with alkyl groups. For example, this molecule
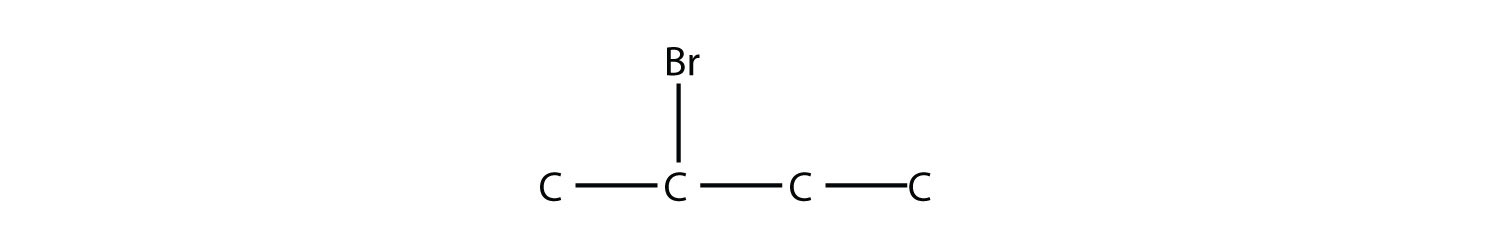
is 2-bromobutane, while this molecule
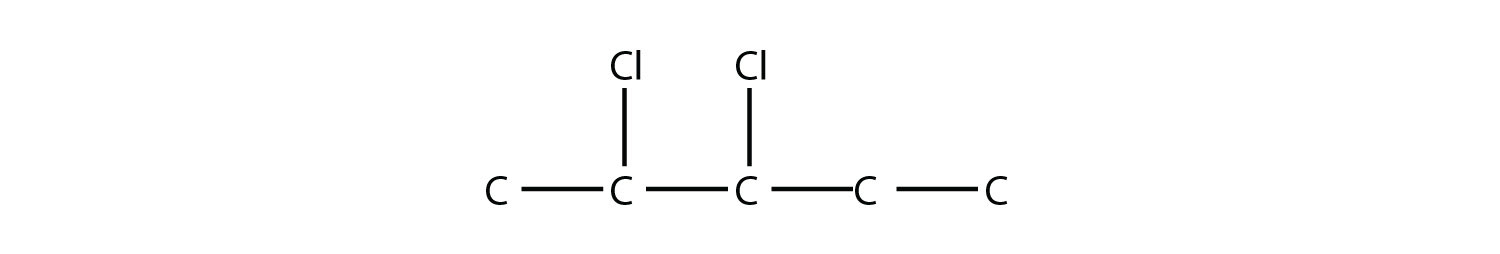
is 2,3-dichloropentane. If alkyl groups are present, the substituents are listed alphabetically. Numerical prefixes are ignored when determining the alphabetical ordering of substituent groups.
Example 6
Name this molecule.
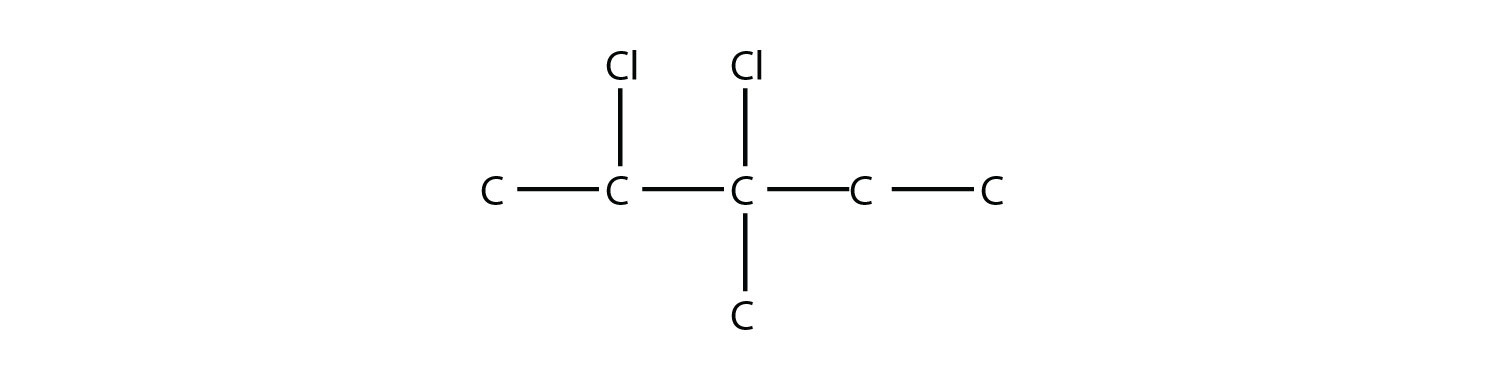
Solution
The longest carbon chain has five C atoms, so the molecule is a pentane. There are two chlorine substituents located on the second and third C atoms, with a one-carbon methyl group on the third C atom as well. The correct name for this molecule is 2,3-dichloro-3-methylpentane.
Test Yourself
Name this molecule.
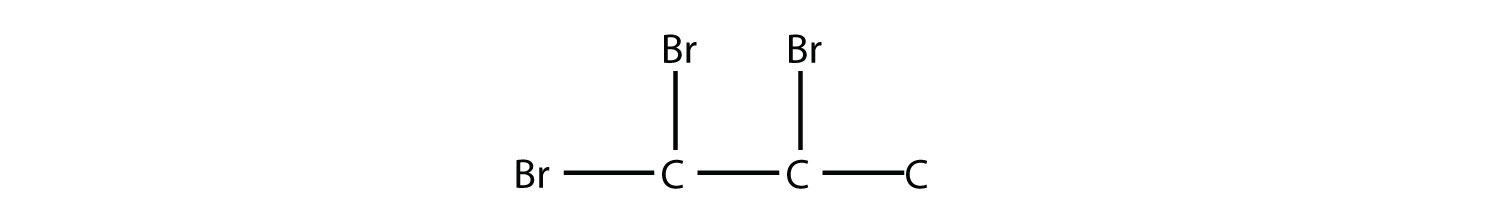
Answer
1,1,2-tribromopropane
Another simple functional group is the covalently bonded OH group. This is the alcoholAn organic compound that contains an OH functional group. functional group. It is not the hydroxide ion; rather than being present as a negatively charged species, in organic chemistry it is a covalently bonded functional group.
Like alkyl halides, alcohols have a common naming system and a more formal system. The common system is similar to that of alkyl halides: name the alkyl group attached to the OH group, ending with the suffix -yl, and add the word alcohol as a second word. So CH3OH is methyl alcohol; CH3CH2OH is ethyl alcohol, and CH3CH2CH2OH is propyl alcohol.
As with alkyl halides, though, this system is limited (although for smaller alcohols, it is very common in everyday usage). The formal system of naming uses the name of the hydrocarbon containing the OH group and having the correct number of C atoms, dropping the final -e of the name and appending the suffix -ol. Thus CH3OH is methanol and CH3CH2OH is ethanol. For larger alcohol molecules, we use a number to indicate the position of the OH group on the longest carbon chain, similar to the number needed for alkenes and alkynes. Again, the carbon chain is numbered to give the OH group the lowest number, no matter how large the other numbers are. So CH3CH2CH2OH is 1-propanol, while CH3CHOHCH3 is 2-propanol. (A common component in many medicine cabinets, 2‑propanol is also known as isopropanol or isopropyl alcohol [Figure 16.3 "Isopropyl Alcohol"]).
Figure 16.3 Isopropyl Alcohol

What you find labeled isopropyl alcohol in a medicine cabinet is more formally called 2-propanol.
Source: Photo courtesy of Craig Spurrier, http://en.wikipedia.org/wiki/File:Rubbing_alcohol.JPG.
Another acceptable way of naming an alcohol—especially a more complicated molecule—is to name the OH group as the hydroxy substituent and give it a numerical position like an alkyl group or a halogen atom. Thus 2-propanol would be called 2-hydroxypropane by this convention.
Example 7
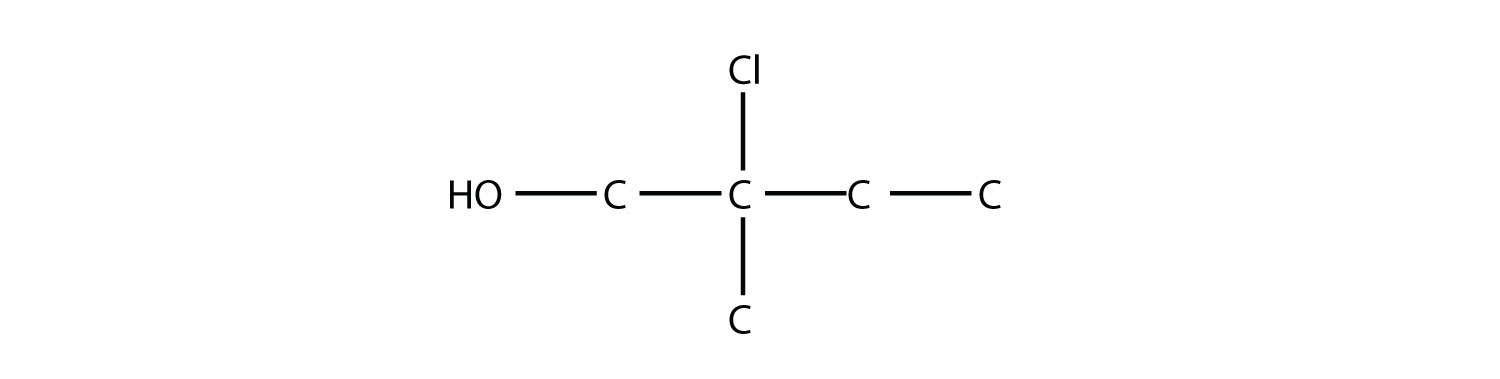
Name this molecule as an alcohol and as a substituted alkane.
Solution
The longest carbon chain containing the OH group has four C atoms, so the parent hydrocarbon is butane. Because the OH group is on the first C atom, it is 1-butanol. There is a methyl group on the second C atom, as well as a Cl atom, so the formal name for this alcohol is 2-chloro-2-methyl-1-butanol. If naming the alcohol group as a substituent, it would be 2-chloro-1-hydroxy-2-methylbutane.
Test Yourself
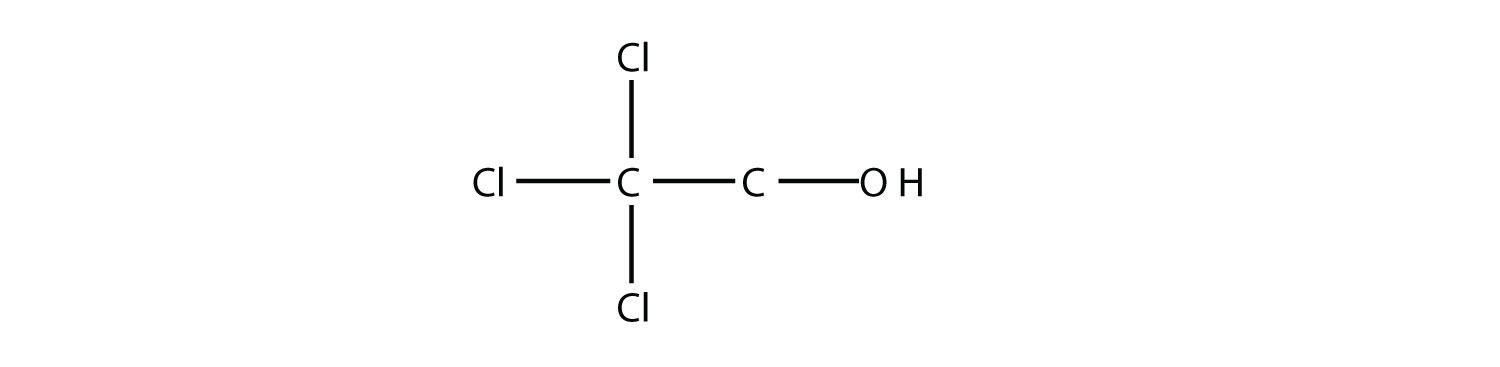
Name this molecule as an alcohol and as a substituted alkane.
Answer
2,2,2-trichloroethanol; 2,2,2-trichloro-1-hydroxyethane
Most alkyl halides are insoluble in H2O. Smaller alcohols, however, are very soluble in H2O because these molecules can engage in hydrogen bonding with H2O molecules. For larger molecules, however, the polar OH group is overwhelmed by the nonpolar alkyl part of the molecule. While methanol is soluble in H2O in all proportions, only about 2.6 g of pentanol will dissolve in 100 g of H2O. Larger alcohols have an even lower solubility in H2O.
One reaction common to alcohols and alkyl halides is eliminationThe removal of an HZ (Z = halogen, OH) from an alkyl halide or an alcohol., the removal of the functional group (either X or OH) and an H atom from an adjacent carbon. The general reaction can be written as follows:
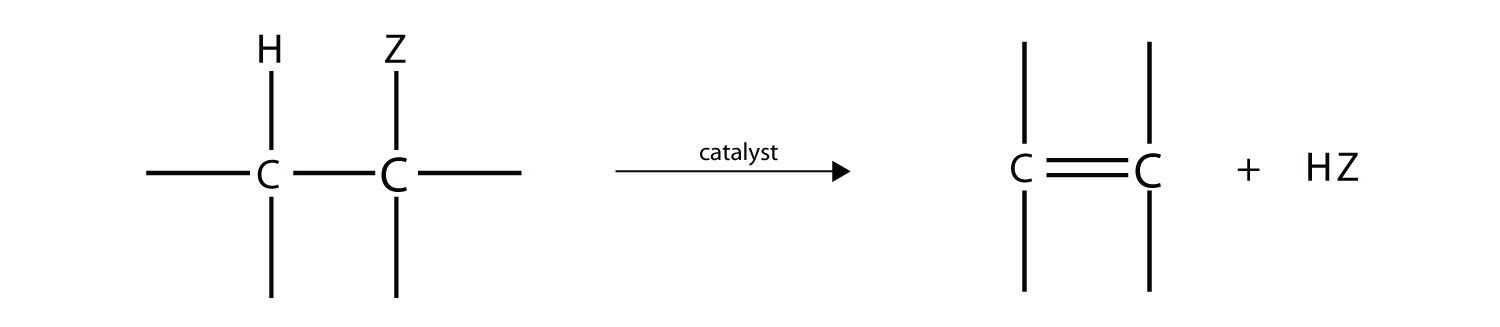
where Z represents either the X or the OH group. The biggest difference between elimination in alkyl halides and elimination in alcohols is the identity of the catalyst: for alkyl halides, the catalyst is a strong base; for alcohols, the catalyst is a strong acid. For compounds in which there are H atoms on more than one adjacent carbon, a mixture of products results.
Example 8
Predict the organic product(s) of this reaction.
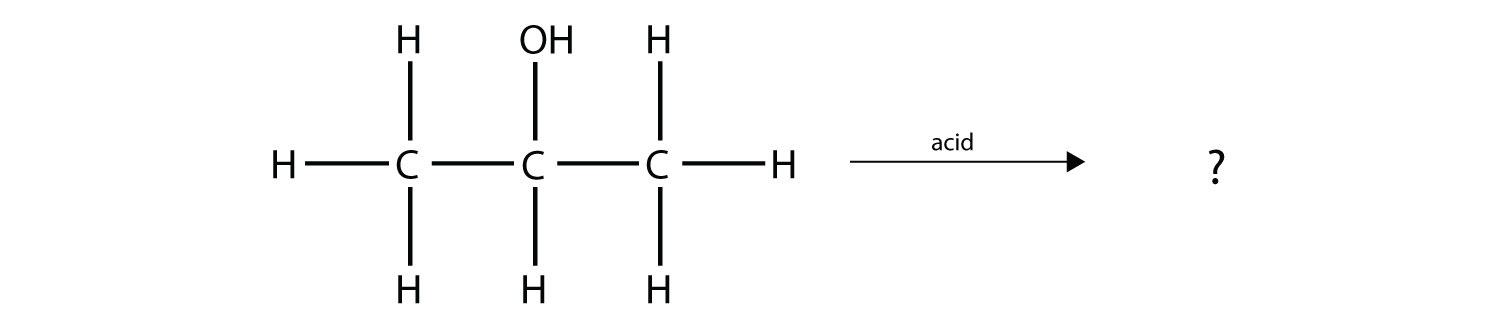
Solution
Under these conditions, an HOH (otherwise known as H2O) molecule will be eliminated, and an alkene will be formed. It does not matter which adjacent carbon loses the H atom; in either case the product will be
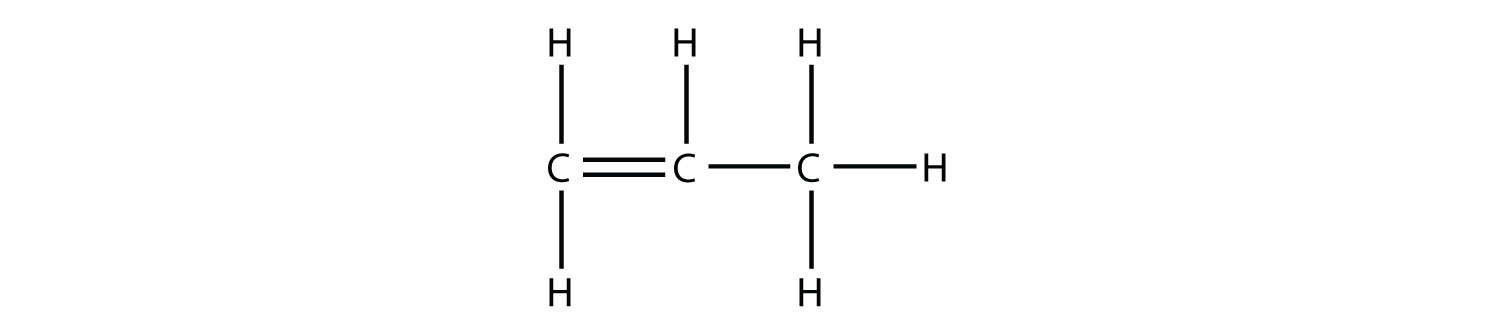
which is propene.
Test Yourself
Predict the organic product(s) of this reaction.
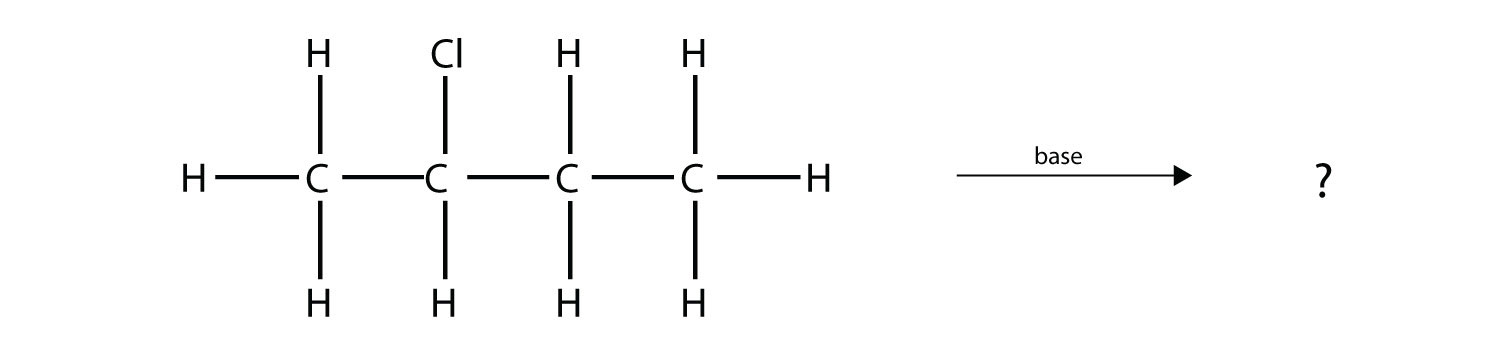
Answer
1-butene and 2-butene
Key Takeaways
- Alkyl halides have a halogen atom as a functional group.
- Alcohols have an OH group as a functional group.
- Nomenclature rules allow us to name alkyl halides and alcohols.
- In an elimination reaction, a double bond is formed as an HX or an HOH molecule is removed.
Exercises
-
Define functional group and give two examples.
-
What is elimination? How does it differ for alkyl halides and alcohols?
-
Name this molecule.
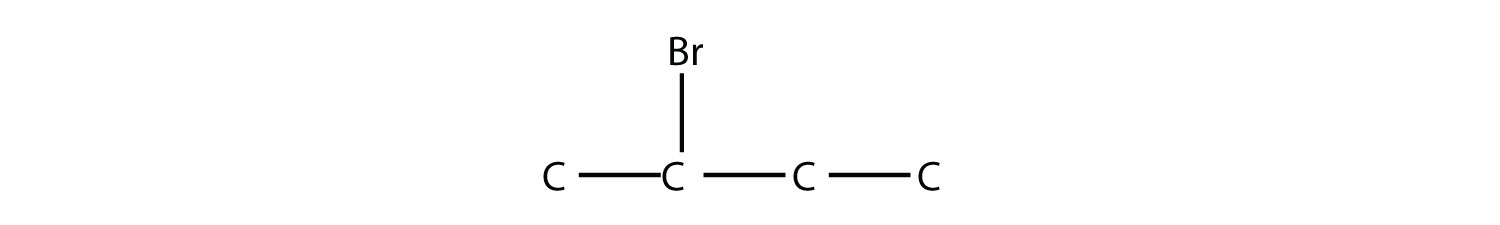
-
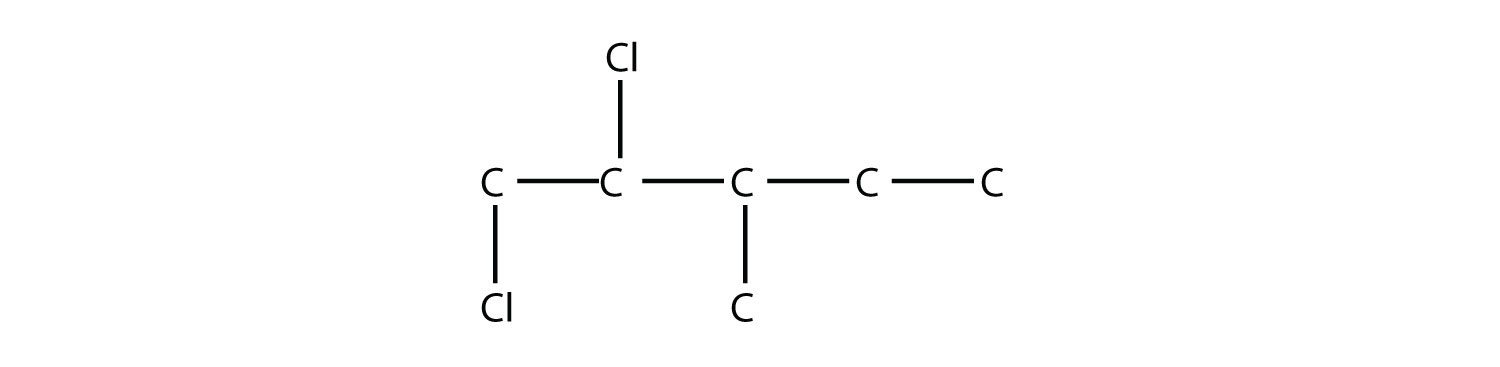
Name this molecule.
-
Name this molecule.
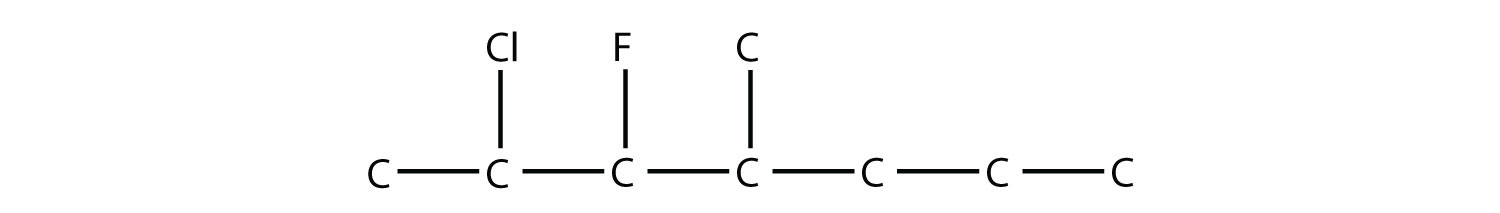
-
Name this molecule.
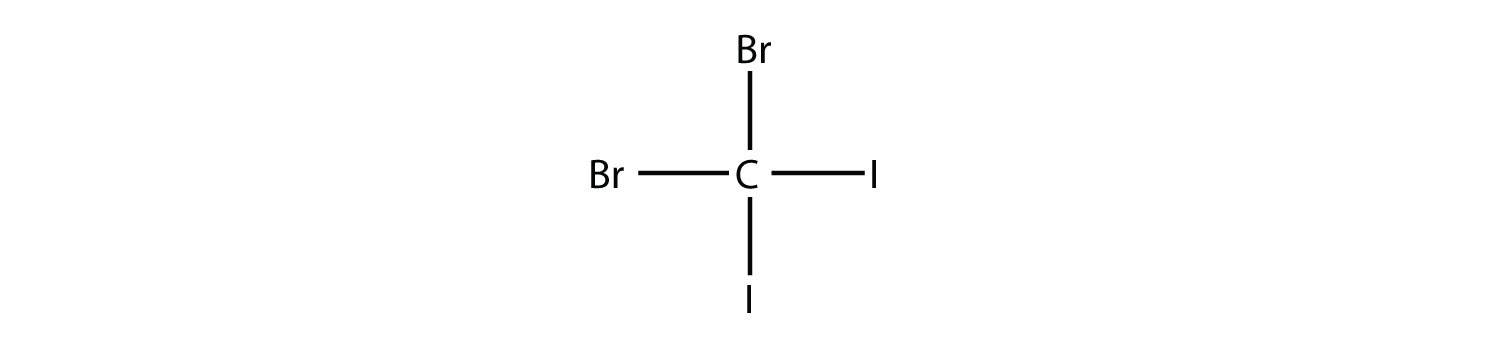
-
Name this molecule.
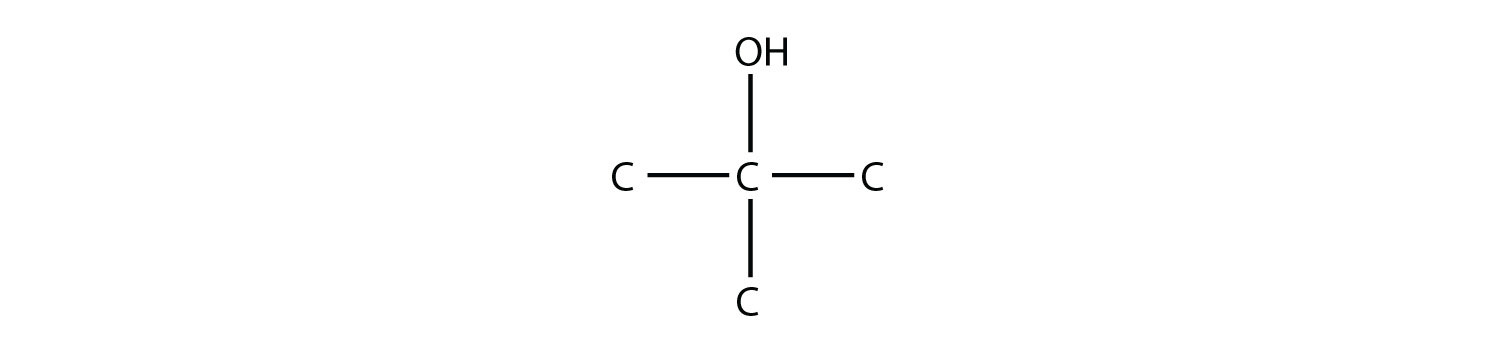
-
Name this molecule.
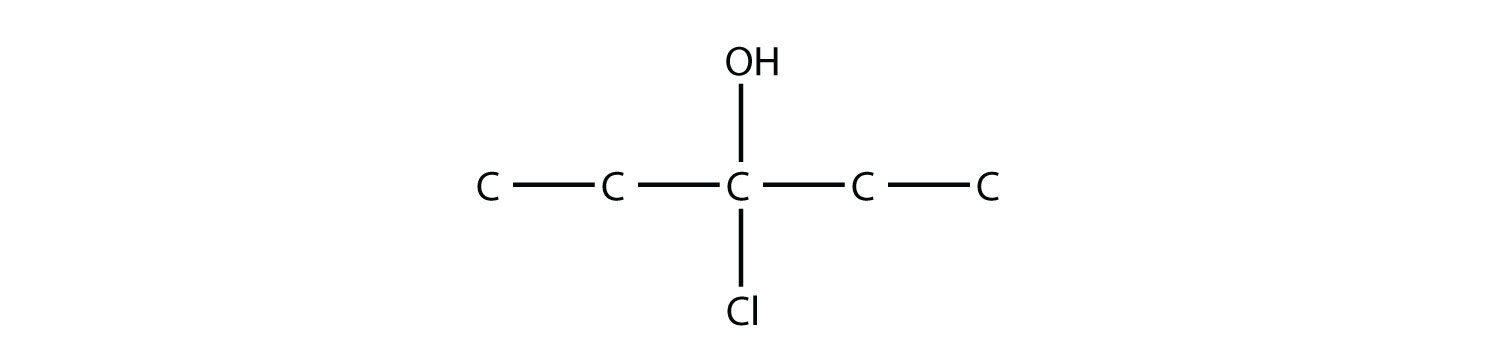
-
Name this molecule.

-
Name this molecule.

-
Predict the product(s) of this elimination reaction.
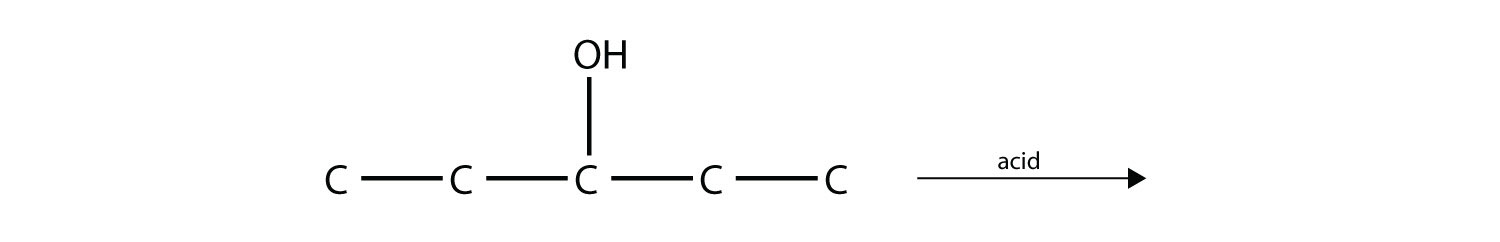
-
Predict the product(s) of this elimination reaction.
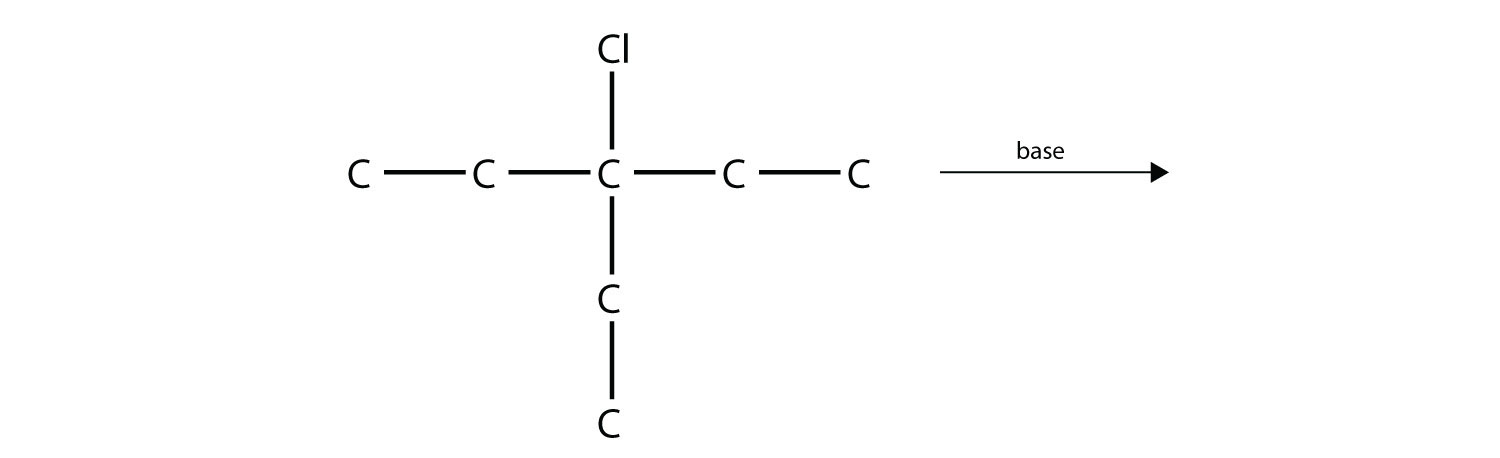
-
Predict the product(s) of this elimination reaction.
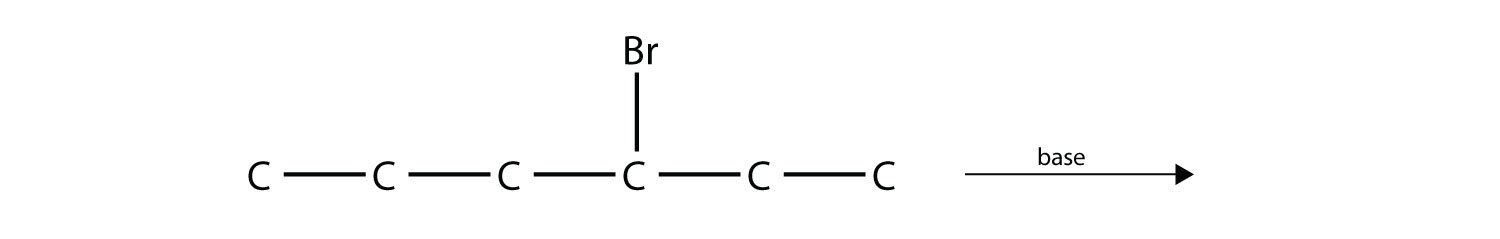
-
Predict the product(s) of this elimination reaction.
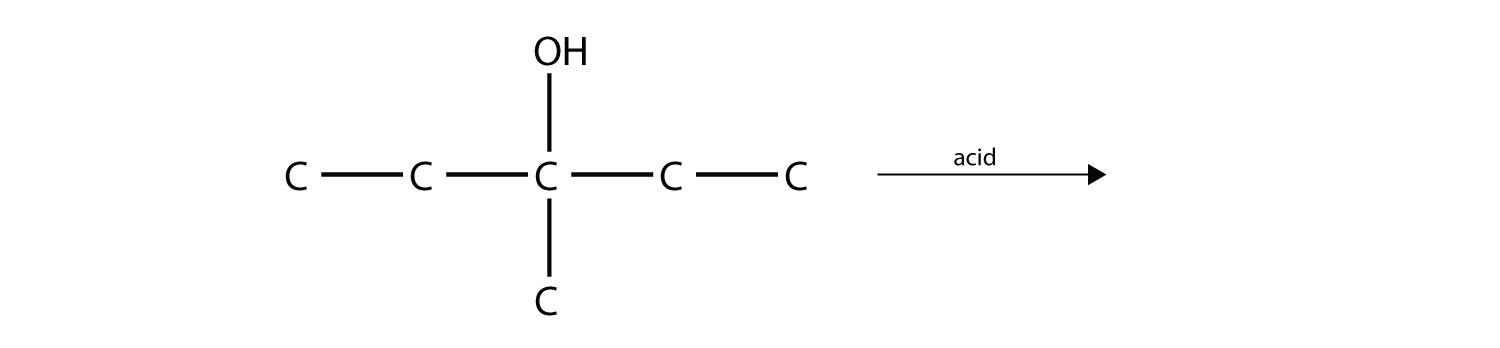
Answers
-
a group of atoms with a certain reactivity; halogen atoms and alcohol groups (answers will vary).
-
-
2-bromobutane
-
-
2-chloro-3-fluoro-4-methylheptane
-
-
2-methyl-2-propanol
-
-
4-octanol
-
-
2-pentene
-
-
2-hexene and 3-hexene
-




