This is “Acids and Bases”, chapter 12 from the book Beginning Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
Chapter 12 Acids and Bases
Opening Essay
Formerly there were rather campy science-fiction television shows in which the hero was always being threatened with death by being plunged into a vat of boiling acid: “Mwa ha ha, Buck Rogers [or whatever the hero’s name was], prepare to meet your doom by being dropped into a vat of boiling acid!” (The hero always escapes, of course.) This may have been interesting drama but not very good chemistry. If the villain knew his/her/its science, the hero would have been dropped into a vat of boiling base.
Recall that the active component of a classic acid is the H+ ion, while the active part of a classic base is the OH− ion. Both ions are related to water in that all H+ ion needs to become a water molecule is an OH− ion, while all an OH− ion needs to become water is an H+ ion. Consider the relative masses involved: an ion of mass 1 needs an ion of mass 17 to make water, while an ion of mass 17 needs an ion of mass 1 to make water. Which process do you think will be easier?
In fact, bases are more potentially dangerous than acids because it is much easier for an OH− ion to rip off an H+ ion from surrounding matter than it is for an H+ ion to rip off an OH− ion. Certain household chemicals, such as some brands of cleanser, can be very concentrated bases, which makes them among the most potentially hazardous substances found around the home; if spilled on the skin, the strong caustic compound can immediately remove H+ ions from the flesh, resulting in chemical burns. Compare that to the fact that we occasionally purposefully ingest substances such as citrus fruits, vinegar, and wine—all of which contain acids. (Of course, some parts of the body, such as the eyes, are extremely sensitive to acids as well as bases.) It seems that our bodies are more capable of dealing with acids than with bases.

On the left is a common acid, and on the right is a common base. Which one is more potentially hazardous?
© Thinkstock
So a note to all the villains out there: get your chemistry right if you want to be successful!
Acids and bases are important classes of chemical compounds. They are part of the foods and beverages we ingest, they are present in medicines and other consumer products, and they are prevalent in the world around us. In this chapter, we will focus on acids and bases and their chemistry.
12.1 Arrhenius Acids and Bases
Learning Objectives
- Identify an Arrhenius acid and an Arrhenius base.
- Write the chemical reaction between an Arrhenius acid and an Arrhenius base.
Historically, the first chemical definition of an acid and a base was put forward by Svante Arrhenius, a Swedish chemist, in 1884. An Arrhenius acidA compound that increases the hydrogen ion concentration in aqueous solution. is a compound that increases the H+ ion concentration in aqueous solution. The H+ ion is just a bare proton, and it is rather clear that bare protons are not floating around in an aqueous solution. Instead, chemistry has defined the hydronium ionThe actual chemical species that represents a hydrogen ion. (H3O+) as the actual chemical species that represents an H+ ion. H+ ions and H3O+ ions are often considered interchangeable when writing chemical equations (although a properly balanced chemical equation should also include the additional H2O). Classic Arrhenius acids can be considered ionic compounds in which H+ is the cation. Table 12.1 "Some Arrhenius Acids" lists some Arrhenius acids and their names.
Table 12.1 Some Arrhenius Acids
| Formula | Name |
|---|---|
| HC2H3O2 (also written CH3COOH) | acetic acid |
| HClO3 | chloric acid |
| HCl | hydrochloric acid |
| HBr | hydrobromic acid |
| HI | hydriodic acid |
| HF | hydrofluoric acid |
| HNO3 | nitric acid |
| H2C2O4 | oxalic acid |
| HClO4 | perchloric acid |
| H3PO4 | phosphoric acid |
| H2SO4 | sulfuric acid |
| H2SO3 | sulfurous acid |
An Arrhenius baseA compound that increases the hydroxide ion concentration in aqueous solution. is a compound that increases the OH− ion concentration in aqueous solution. Ionic compounds of the OH− ion are classic Arrhenius bases.
Example 1
Identify each compound as an Arrhenius acid, an Arrhenius base, or neither.
- HNO3
- CH3OH
- Mg(OH)2
Solution
- This compound is an ionic compound between H+ ions and NO3− ions, so it is an Arrhenius acid.
- Although this formula has an OH in it, we do not recognize the remaining part of the molecule as a cation. It is neither an acid nor a base. (In fact, it is the formula for methanol, an organic compound.)
- This formula also has an OH in it, but this time we recognize that the magnesium is present as Mg2+ cations. As such, this is an ionic compound of the OH− ion and is an Arrhenius base.
Test Yourself
Identify each compound as an Arrhenius acid, an Arrhenius base, or neither.
- KOH
- H2SO4
- C2H6
Answer
- Arrhenius base
- Arrhenius acid
- neither
Acids have some properties in common. They turn litmus, a plant extract, red. They react with some metals to give off H2 gas. They react with carbonate and hydrogen carbonate salts to give off CO2 gas. Acids that are ingested typically have a sour, sharp taste. (The name acid comes from the Latin word acidus, meaning “sour.”) Bases also have some properties in common. They are slippery to the touch, turn litmus blue, and have a bitter flavor if ingested.
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A saltAny ionic compound that is formed from a reaction between an acid and a base., in chemistry, is any ionic compound made by combining an acid with a base. A reaction between an acid and a base is called a neutralization reactionThe reaction of an acid and a base to produce water and a salt. and can be represented as follows:
acid + base → H2O + saltThe stoichiometry of the balanced chemical equation depends on the number of H+ ions in the acid and the number of OH− ions in the base.
Example 2
Write the balanced chemical equation for the neutralization reaction between H2SO4 and KOH. What is the name of the salt that is formed?
Solution
The general reaction is as follows:
H2SO4 + KOH → H2O + saltBecause the acid has two H+ ions in its formula, we need two OH− ions to react with it, making two H2O molecules as product. The remaining ions, K+ and SO42−, make the salt potassium sulfate (K2SO4). The balanced chemical reaction is as follows:
H2SO4 + 2KOH → 2H2O + K2SO4Test Yourself
Write the balanced chemical equation for the neutralization reaction between HCl and Mg(OH)2. What is the name of the salt that is formed?
Answer
2HCl + Mg(OH)2 → 2H2O + MgCl2; magnesium chloride
Key Takeaways
- An Arrhenius acid is a compound that increases the H+ ion concentration in aqueous solution.
- An Arrhenius base is a compound that increases the OH− ion concentration in aqueous solution.
- The reaction between an Arrhenius acid and an Arrhenius base is called neutralization and results in the formation of water and a salt.
Exercises
-
Define Arrhenius acid.
-
Define Arrhenius base.
-
What are some general properties of Arrhenius acids?
-
What are some general properties of Arrhenius bases?
-
Identify each substance as an Arrhenius acid, an Arrhenius base, or neither.
- NaOH
- C2H5OH
- H3PO4
-
Identify each substance as an Arrhenius acid, an Arrhenius base, or neither.
- C6H12O6
- HNO2
- Ba(OH)2
-
Write the balanced chemical equation for the neutralization reaction between KOH and H2C2O4. What is the salt?
-
Write the balanced chemical equation for the neutralization reaction between Sr(OH)2 and H3PO4. What is the salt?
-
Write the balanced chemical equation for the neutralization reaction between HCl and Fe(OH)3. What is the salt?
-
Write the balanced chemical equation for the neutralization reaction between H2SO4 and Cr(OH)3. What is the salt?
-
CaCl2 would be the product of the reaction of what acid and what base?
-
Zn(NO3)2 would be product of the reaction of what acid and what base?
-
BaSO4 would be product of the reaction of what acid and what base?
-
Na3PO4 would be product of the reaction of what acid and what base?
Answers
-
a compound that increases the H+ concentration in water
-
-
sour taste, react with metals, and turn litmus red
-
-
- Arrhenius base
- neither
- Arrhenius acid
-
-
2KOH + H2C2O4 → 2H2O + K2C2O4; K2C2O4
-
-
3HCl + Fe(OH)3 → 3H2O + FeCl3; FeCl3
-
-
HCl and Ca(OH)2
-
-
H2SO4 and Ba(OH)2
-
12.2 Brønsted-Lowry Acids and Bases
Learning Objectives
- Identify a Brønsted-Lowry acid and a Brønsted-Lowry base.
- Identify conjugate acid-base pairs in an acid-base reaction.
The Arrhenius definition of acid and base is limited to aqueous (that is, water) solutions. Although this is useful because water is a common solvent, it is limited to the relationship between the H+ ion and the OH− ion. What would be useful is a more general definition that would be more applicable to other chemical reactions and, importantly, independent of H2O.
In 1923, Danish chemist Johannes Brønsted and English chemist Thomas Lowry independently proposed new definitions for acids and bases, ones that focus on proton transfer. A Brønsted-Lowry acidAny species that can donate a proton to another molecule. is any species that can donate a proton (H+) to another molecule. A Brønsted-Lowry baseAny species that can accept a proton from another molecule. is any species that can accept a proton from another molecule. In short, a Brønsted-Lowry acid is a proton donor (PD), while a Brønsted-Lowry base is a proton acceptor (PA).
It is easy to see that the Brønsted-Lowry definition covers the Arrhenius definition of acids and bases. Consider the prototypical Arrhenius acid-base reaction:
The acid species and base species are marked. The proton, however, is (by definition) a proton donor (labeled PD), while the OH− ion is acting as the proton acceptor (labeled PA):
The proton donor is a Brønsted-Lowry acid, and the proton acceptor is the Brønsted-Lowry base:
Thus H+ is an acid by both definitions, and OH− is a base by both definitions.
Ammonia (NH3) is a base even though it does not contain OH− ions in its formula. Instead, it generates OH− ions as the product of a proton-transfer reaction with H2O molecules; NH3 acts like a Brønsted-Lowry base, and H2O acts like a Brønsted-Lowry acid:
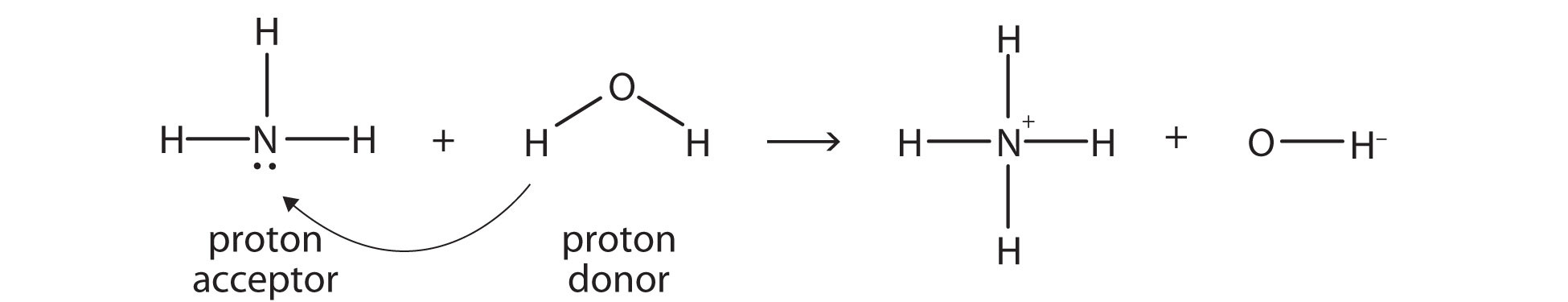
A reaction with water is called hydrolysisA reaction with water.; we say that NH3 hydrolyzes to make NH4+ ions and OH− ions.
Even the dissolving of an Arrhenius acid in water can be considered a Brønsted-Lowry acid-base reaction. Consider the process of dissolving HCl(g) in water to make an aqueous solution of hydrochloric acid. The process can be written as follows:
HCl(g) + H2O(ℓ) → H3O+(aq) + Cl−(aq)HCl(g) is the proton donor and therefore a Brønsted-Lowry acid, while H2O is the proton acceptor and a Brønsted-Lowry base. These two examples show that H2O can act as both a proton donor and a proton acceptor, depending on what other substance is in the chemical reaction. A substance that can act as a proton donor or a proton acceptor is called amphiproticA substance that can act as a proton donor or a proton acceptor.. Water is probably the most common amphiprotic substance we will encounter, but other substances are also amphiprotic.
Example 3
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
C6H5OH + NH2− → C6H5O− + NH3Solution
The C6H5OH molecule is losing an H+; it is the proton donor and the Brønsted-Lowry acid. The NH2− ion (called the amide ion) is accepting the H+ ion to become NH3, so it is the Brønsted-Lowry base.
Test Yourself
Identify the Brønsted-Lowry acid and the Brønsted-Lowry base in this chemical equation.
Al(H2O)63+ + H2O → Al(H2O)5(OH)2+ + H3O+Answer
Brønsted-Lowry acid: Al(H2O)63+; Brønsted-Lowry base: H2O
In the reaction between NH3 and H2O,
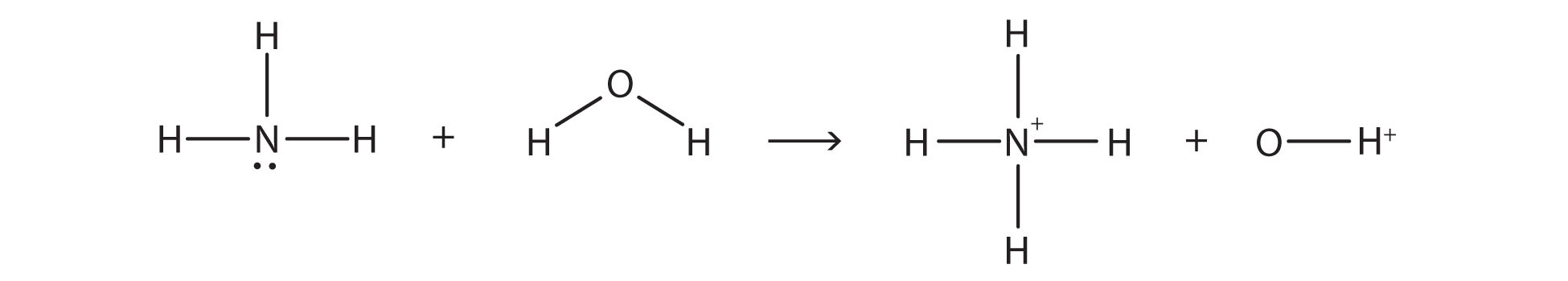
the chemical reaction does not go to completion; rather, the reverse process occurs as well, and eventually the two processes cancel out any additional change. At this point, we say the chemical reaction is at equilibrium. Both processes still occur, but any net change by one process is countered by the same net change by the other process; it is a dynamic, rather than a static, equilibrium. Because both reactions are occurring, it makes sense to use a double arrow instead of a single arrow:
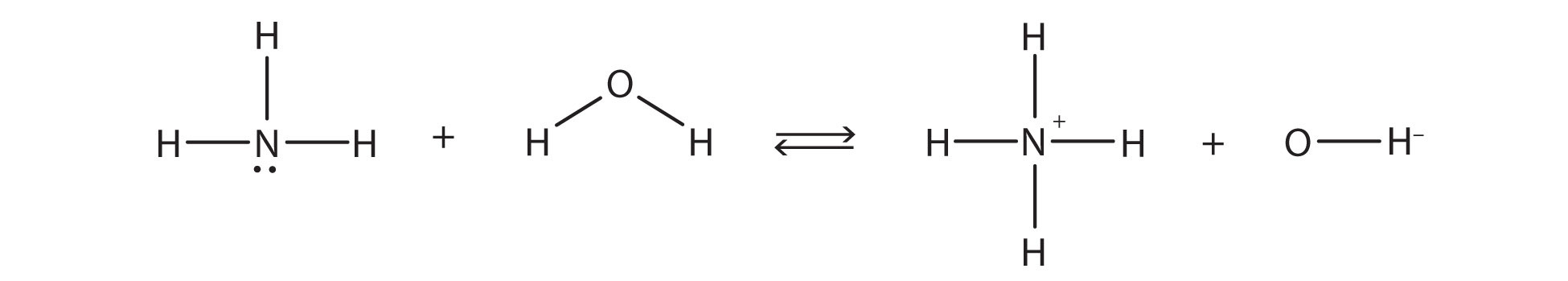
What do you notice about the reverse reaction? The NH4+ ion is donating a proton to the OH− ion, which is accepting it. This means that the NH4+ ion is acting as the proton donor, or Brønsted-Lowry acid, while OH− ion, the proton acceptor, is acting as a Brønsted-Lowry base. The reverse reaction is also a Brønsted-Lowry acid base reaction:
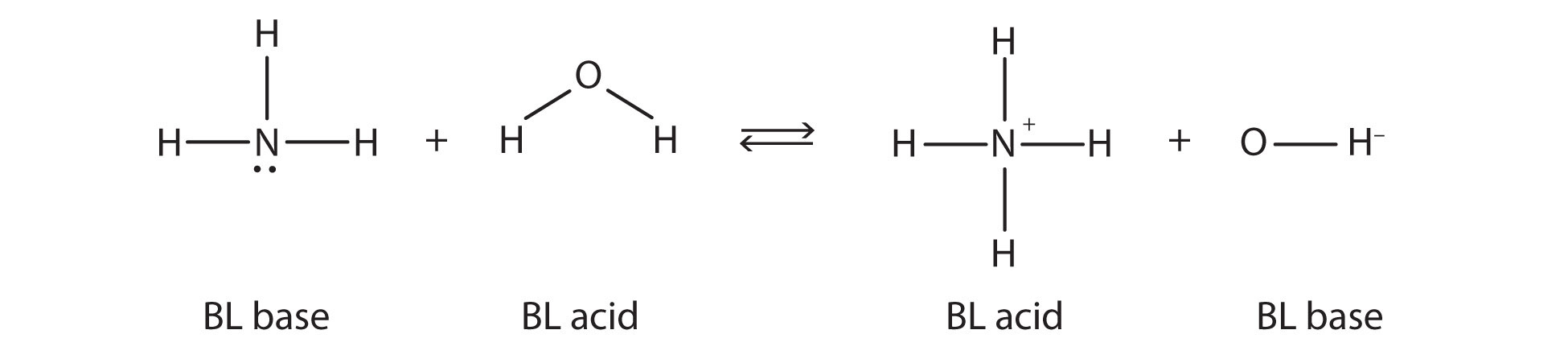
This means that both reactions are acid-base reactions by the Brønsted-Lowry definition. If you consider the species in this chemical reaction, two sets of similar species exist on both sides. Within each set, the two species differ by a proton in their formulas, and one member of the set is a Brønsted-Lowry acid, while the other member is a Brønsted-Lowry base. These sets are marked here:

The two sets—NH3/NH4+ and H2O/OH−—are called conjugate acid-base pairsTwo species whose formulas differ by only a hydrogen ion.. We say that NH4+ is the conjugate acid of NH3, OH− is the conjugate base of H2O, and so forth. Every Brønsted-Lowry acid-base reaction can be labeled with two conjugate acid-base pairs.
Example 4
Identify the conjugate acid-base pairs in this equilibrium.
Solution
One pair is H2O and OH−, where H2O has one more H+ and is the conjugate acid, while OH− has one less H+ and is the conjugate base. The other pair consists of (CH3)3N and (CH3)3NH+, where (CH3)3NH+ is the conjugate acid (it has an additional proton) and (CH3)3N is the conjugate base.
Test Yourself
Identify the conjugate acid-base pairs in this equilibrium.
Answer
H2O (acid) and OH− (base); NH2− (base) and NH3 (acid)
Chemistry Is Everywhere: Household Acids and Bases
Many household products are acids or bases. For example, the owner of a swimming pool may use muriatic acid to clean the pool. Muriatic acid is another name for HCl(aq). In Chapter 4 "Chemical Reactions and Equations", Section 4.5 "Neutralization Reactions", vinegar was mentioned as a dilute solution of acetic acid [HC2H3O2(aq)]. In a medicine chest, one may find a bottle of vitamin C tablets; the chemical name of vitamin C is ascorbic acid (HC6H7O6).
One of the more familiar household bases is NH3, which is found in numerous cleaning products. NH3 is a base because it increases the OH− ion concentration by reacting with H2O:
NH3(aq) + H2O(ℓ) → NH4+(aq) + OH−(aq)Many soaps are also slightly basic because they contain compounds that act as Brønsted-Lowry bases, accepting protons from H2O and forming excess OH− ions. This is one explanation for why soap solutions are slippery.
Perhaps the most dangerous household chemical is the lye-based drain cleaner. Lye is a common name for NaOH, although it is also used as a synonym for KOH. Lye is an extremely caustic chemical that can react with grease, hair, food particles, and other substances that may build up and clog a water pipe. Unfortunately, lye can also attack body tissues and other substances in our bodies. Thus when we use lye-based drain cleaners, we must be very careful not to touch any of the solid drain cleaner or spill the water it was poured into. Safer, nonlye drain cleaners (like the one in the accompanying figure) use peroxide compounds to react on the materials in the clog and clear the drain.

Drain cleaners can be made from a reactive material that is less caustic than a base.
Source: Photo used by permission of Citrasolv, LLC.
Key Takeaways
- A Brønsted-Lowry acid is a proton donor; a Brønsted-Lowry base is a proton acceptor.
- Acid-base reactions include two sets of conjugate acid-base pairs.
Exercises
-
Define Brønsted-Lowry acid. How does it differ from an Arrhenius acid?
-
Define Brønsted-Lowry base. How does it differ from an Arrhenius base?
-
Write the dissociation of hydrogen bromide in water as a Brønsted-Lowry acid-base reaction and identify the proton donor and proton acceptor.
-
Write the dissociation of nitric acid in water as a Brønsted-Lowry acid-base reaction and identify the proton donor and proton acceptor.
-
Pyridine (C5H5N) acts as a Brønsted-Lowry base in water. Write the hydrolysis reaction for pyridine and identify the Brønsted-Lowry acid and Brønsted-Lowry base.
-
The methoxide ion (CH3O−) acts as a Brønsted-Lowry base in water. Write the hydrolysis reaction for the methoxide ion and identify the Brønsted-Lowry acid and Brønsted-Lowry base.
-
Identify the Brønsted-Lowry acid and Brønsted-Lowry base in this chemical equation.
H3PO4 + OH− → H2PO4− + H2O -
Identify the Brønsted-Lowry acid and Brønsted-Lowry base in this chemical equation.
H2C2O4 + 2F− → 2HF + C2O42− -
Predict the products of this reaction, assuming it undergoes a Brønsted-Lowry acid-base reaction.
HC2H3O2 + C5H5N → ? -
Predict the products of this reaction, assuming it undergoes a Brønsted-Lowry acid-base reaction.
(C2H5)3N + H2O → ? -
What is the conjugate acid of H2O? of NH3?
-
What is the conjugate acid of H2PO4−? of NO3−?
-
What is the conjugate base of HSO4−? of H2O?
-
What is the conjugate base of H3O+? of H2SO4?
-
Identify the conjugate acid-base pairs in this reaction.
HSO4− + PO43− → SO42− + HPO42− -
Identify the conjugate acid-base pairs in this reaction.
HClO3 + (C2H5)3N → ClO3− + (C2H5)3NH+ -
Identify the conjugate acid-base pairs in this reaction.
NH3 + C6H5O− → C6H5OH + NH2− -
Identify the conjugate acid-base pairs in this reaction.
C5H5NH+ + C2O42− → C5H5N + HC2O4−
Answers
-
A Brønsted-Lowry acid is a proton donor. It does not necessarily increase the H+ concentration in water.
-
-
HBr + H2O → H3O+ + Br−; PD: HBr; PA: H2O
-
-
C5H5N + H2O → C5H5NH+ + OH−; PD: H2O; PA: C5H5N
-
-
BL acid: H3PO4; BL base: OH−
-
-
C2H3O2− and C5H5NH+
-
-
H3O+; NH4+
-
-
SO42−; OH−
-
-
HSO4− and SO42−; PO43− and HPO42−
-
-
NH3 and NH2−; C6H5O− and C6H5OH
-
12.3 Acid-Base Titrations
Learning Objectives
- Describe a titration experiment.
- Explain what an indicator does.
- Perform a titration calculation correctly.
The reaction of an acid with a base to make a salt and water is a common reaction in the laboratory, partly because so many compounds can act as acids or bases. Another reason that acid-base reactions are so prevalent is because they are often used to determine quantitative amounts of one or the other. Performing chemical reactions quantitatively to determine the exact amount of a reagent is called a titrationA chemical reaction performed quantitatively to determine the exact amount of a reagent.. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. Here, we will consider titrations that involve acid-base reactions.
In a titration, one reagent has a known concentration or amount, while the other reagent has an unknown concentration or amount. Typically, the known reagent (the titrantThe reagent of known concentration.) is added to the unknown quantity and is dissolved in solution. The unknown amount of substance (the analyteThe reagent of unknown concentration.) may or may not be dissolved in solution (but usually is). The titrant is added to the analyte using a precisely calibrated volumetric delivery tube called a burette (also spelled buret; see Figure 12.1 "Equipment for Titrations"). The burette has markings to determine how much volume of solution has been added to the analyte. When the reaction is complete, it is said to be at the equivalence pointThe point of the reaction when all the analyte has been reacted with the titrant.; the number of moles of titrant can be calculated from the concentration and the volume, and the balanced chemical equation can be used to determine the number of moles (and then concentration or mass) of the unknown reactant.
Figure 12.1 Equipment for Titrations
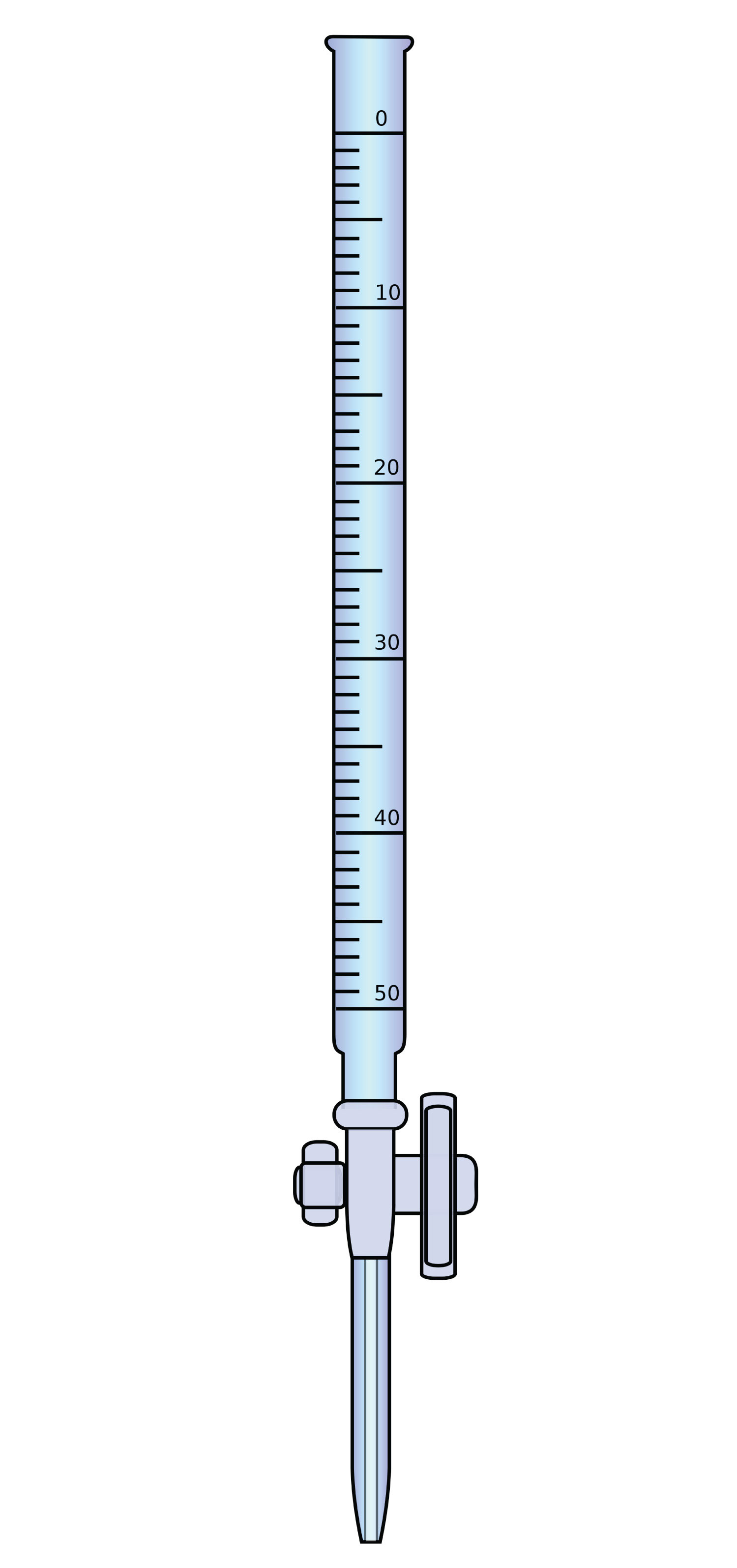
A burette is a type of liquid dispensing system that can accurately indicate the volume of liquid dispensed.
For example, suppose 25.66 mL (or 0.02566 L) of 0.1078 M HCl was used to titrate an unknown sample of NaOH. What mass of NaOH was in the sample? We can calculate the number of moles of HCl reacted:
# mol HCl = (0.02566 L)(0.1078 M) = 0.002766 mol HClWe also have the balanced chemical reaction between HCl and NaOH:
HCl + NaOH → NaCl + H2OSo we can construct a conversion factor to convert to number of moles of NaOH reacted:
Then we convert this amount to mass, using the molar mass of NaOH (40.00 g/mol):
This is type of calculation is performed as part of a titration.
Example 5
What mass of Ca(OH)2 is present in a sample if it is titrated to its equivalence point with 44.02 mL of 0.0885 M HNO3? The balanced chemical equation is as follows:
2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2OSolution
In liters, the volume is 0.04402 L. We calculate the number of moles of titrant:
# moles HNO3 = (0.04402 L)(0.0885 M) = 0.00390 mol HNO3Using the balanced chemical equation, we can determine the number of moles of Ca(OH)2 present in the analyte:
Then we convert this to a mass using the molar mass of Ca(OH)2:
Test Yourself
What mass of H2C2O4 is present in a sample if it is titrated to its equivalence point with 18.09 mL of 0.2235 M NaOH? The balanced chemical reaction is as follows:
H2C2O4 + 2NaOH → Na2C2O4 + 2H2OAnswer
0.182 g
How does one know if a reaction is at its equivalence point? Usually, the person performing the titration adds a small amount of an indicatorA substance whose color change indicates the equivalence point of a titration., a substance that changes color depending on the acidity or basicity of the solution. Because different indicators change colors at different levels of acidity, choosing the correct one is important in performing an accurate titration.
Key Takeaways
- A titration is the quantitative reaction of an acid and a base.
- Indicators are used to show that all the analyte has reacted with the titrant.
Exercises
-
Define titration.
-
What is the difference between the titrant and the analyte?
-
True or false: An acid is always the titrant. Explain your answer.
-
True or false: An analyte is always dissolved before reaction. Explain your answer.
-
If 55.60 mL of 0.2221 M HCl was needed to titrate a sample of NaOH to its equivalence point, what mass of NaOH was present?
-
If 16.33 mL of 0.6664 M KOH was needed to titrate a sample of HC2H3O2 to its equivalence point, what mass of HC2H3O2 was present?
-
It takes 45.66 mL of 0.1126 M HBr to titrate 25.00 mL of Ca(OH)2 to its equivalence point. What is the original concentration of the Ca(OH)2 solution?
-
It takes 9.77 mL of 0.883 M H2SO4 to titrate 15.00 mL of KOH to its equivalence point. What is the original concentration of the KOH solution?
Answers
-
a chemical reaction performed in a quantitative fashion
-
-
False; a base can be a titrant, or the reaction being performed may not even be an acid-base reaction.
-
-
0.494 g
-
-
0.1028 M
-
12.4 Strong and Weak Acids and Bases and Their Salts
Learning Objectives
- Define a strong and a weak acid and base.
- Recognize an acid or a base as strong or weak.
- Determine if a salt produces an acidic or a basic solution.
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction. However, acids can be very different in a very important way. Consider HCl(aq). When HCl is dissolved in H2O, it completely dissociates into H+(aq) and Cl−(aq) ions; all the HCl molecules become ions:
Any acid that dissociates 100% into ions is called a strong acidAny acid that is 100% dissociated into ions in aqueous solution.. If it does not dissociate 100%, it is a weak acidAny acid that is less than 100% dissociated into ions in aqueous solution.. HC2H3O2 is an example of a weak acid:
Because this reaction does not go 100% to completion, it is more appropriate to write it as an equilibrium:
As it turns out, there are very few strong acids, which are given in Table 12.2 "Strong Acids and Bases". If an acid is not listed here, it is a weak acid. It may be 1% ionized or 99% ionized, but it is still classified as a weak acid.
The issue is similar with bases: a strong baseAny base that is 100% dissociated into ions in aqueous solution. is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak baseAny base that is less than 100% dissociated into ions in aqueous solution.. There are very few strong bases (see Table 12.2 "Strong Acids and Bases"); any base not listed is a weak base. All strong bases are OH– compounds. So a base based on some other mechanism, such as NH3 (which does not contain OH− ions as part of its formula), will be a weak base.
Table 12.2 Strong Acids and Bases
| Acids | Bases |
|---|---|
| HCl | LiOH |
| HBr | NaOH |
| HI | KOH |
| HNO3 | RbOH |
| H2SO4 | CsOH |
| HClO3 | Mg(OH)2 |
| HClO4 | Ca(OH)2 |
| Sr(OH)2 | |
| Ba(OH)2 |
Example 6
Identify each acid or base as strong or weak.
- HCl
- Mg(OH)2
- C5H5N
Solution
- Because HCl is listed in Table 12.2 "Strong Acids and Bases", it is a strong acid.
- Because Mg(OH)2 is listed in Table 12.2 "Strong Acids and Bases", it is a strong base.
- The nitrogen in C5H5N would act as a proton acceptor and therefore can be considered a base, but because it does not contain an OH compound, it cannot be considered a strong base; it is a weak base.
Test Yourself
Identify each acid or base as strong or weak.
- RbOH
- HNO2
Answers
- strong base
- weak acid
Example 7
Write the balanced chemical equation for the dissociation of Ca(OH)2 and indicate whether it proceeds 100% to products or not.
Solution
This is an ionic compound of Ca2+ ions and OH− ions. When an ionic compound dissolves, it separates into its constituent ions:
Ca(OH)2 → Ca2+(aq) + 2OH−(aq)Because Ca(OH)2 is listed in Table 12.2 "Strong Acids and Bases", this reaction proceeds 100% to products.
Test Yourself
Write the balanced chemical equation for the dissociation of hydrazoic acid (HN3) and indicate whether it proceeds 100% to products or not.
Answer
The reaction is as follows:
HN3 → H+(aq) + N3−(aq)It does not proceed 100% to products because hydrazoic acid is not a strong acid.
Certain salts will also affect the acidity or basicity of aqueous solutions because some of the ions will undergo hydrolysis, just like NH3 does to make a basic solution. The general rule is that salts with ions that are part of strong acids or bases will not hydrolyze, while salts with ions that are part of weak acids or bases will hydrolyze.
Consider NaCl. When it dissolves in an aqueous solution, it separates into Na+ ions and Cl− ions:
NaCl → Na+(aq) + Cl−(aq)Will the Na+(aq) ion hydrolyze? If it does, it will interact with the OH− ion to make NaOH:
Na+(aq) + H2O → NaOH + H+(aq)However, NaOH is a strong base, which means that it is 100% ionized in solution:
NaOH → Na+(aq) + OH−(aq)The free OH−(aq) ion reacts with the H+(aq) ion to remake a water molecule:
H+(aq) + OH−(aq) → H2OThe net result? There is no change, so there is no effect on the acidity or basicity of the solution from the Na+(aq) ion. What about the Cl− ion? Will it hydrolyze? If it does, it will take an H+ ion from a water molecule:
Cl−(aq) + H2O → HCl + OH−However, HCl is a strong acid, which means that it is 100% ionized in solution:
HCl → H+(aq) + Cl−(aq)The free H+(aq) ion reacts with the OH−(aq) ion to remake a water molecule:
H+(aq) + OH−(aq) → H2OThe net result? There is no change, so there is no effect on the acidity or basicity of the solution from the Cl−(aq) ion. Because neither ion in NaCl affects the acidity or basicity of the solution, NaCl is an example of a neutral saltAn ionic compound that does not affect the acidity of its aqueous solution..
Things change, however, when we consider a salt like NaC2H3O2. We already know that the Na+ ion won’t affect the acidity of the solution. What about the acetate ion? If it hydrolyzes, it will take an H+ from a water molecule:
C2H3O2−(aq) + H2O → HC2H3O2 + OH−(aq)Does this happen? Yes, it does. Why? Because HC2H3O2 is a weak acid. Any chance a weak acid has to form, it will (the same with a weak base). As some C2H3O2− ions hydrolyze with H2O to make the molecular weak acid, OH− ions are produced. OH− ions make solutions basic. Thus NaC2H3O2 solutions are slightly basic, so such a salt is called a basic saltAn ionic compound whose aqueous solution is slightly basic..
There are also salts whose aqueous solutions are slightly acidic. NH4Cl is an example. When NH4Cl is dissolved in H2O, it separates into NH4+ ions and Cl− ions. We have already seen that the Cl− ion does not hydrolyze. However, the NH4+ ion will:
NH4+(aq) + H2O → NH3(aq) + H3O+(aq)Recall from Section 12.1 "Arrhenius Acids and Bases" that H3O+ ion is the hydronium ion, the more chemically proper way to represent the H+ ion. This is the classic acid species in solution, so a solution of NH4+(aq) ions is slightly acidic. NH4Cl is an example of an acid saltAn ionic compound whose aqueous solution is slightly acidic.. The molecule NH3 is a weak base, and it will form when it can, just like a weak acid will form when it can.
So there are two general rules: (1) If an ion derives from a strong acid or base, it will not affect the acidity of the solution. (2) If an ion derives from a weak acid, it will make the solution basic; if an ion derives from a weak base, it will make the solution acidic.
Example 8
Identify each salt as acidic, basic, or neutral.
- KCl
- KNO2
- NH4Br
Solution
- The ions from KCl derive from a strong acid (HCl) and a strong base (KOH). Therefore, neither ion will affect the acidity of the solution, so KCl is a neutral salt.
- Although the K+ ion derives from a strong base (KOH), the NO2− ion derives from a weak acid (HNO2). Therefore the solution will be basic, and KNO2 is a basic salt.
- Although the Br− ions derive from a strong acid (HBr), the NH4+ ion derives from a weak base (NH3), so the solution will be acidic, and NH4Br is an acidic salt.
Test Yourself
Identify each salt as acidic, basic, or neutral.
- (C5H5NH)Cl
- Na2SO3
Answers
- acidic
- basic
Some salts are composed of ions that come from both weak acids and weak bases. The overall effect on an aqueous solution depends on which ion exerts more influence on the overall acidity. We will not consider such salts here.
Key Takeaways
- Strong acids and bases are 100% ionized in aqueous solution.
- Weak acids and bases are less than 100% ionized in aqueous solution.
- Salts of weak acids or bases can affect the acidity or basicity of their aqueous solutions.
Exercises
-
Differentiate between a strong acid and a weak acid.
-
Differentiate between a strong base and a weak base.
-
Identify each as a strong acid or a weak acid. Assume aqueous solutions.
- HF
- HCl
- HC2O4
-
Identify each as a strong base or a weak base. Assume aqueous solutions.
- NaOH
- Al(OH)3
- C4H9NH2
-
Write a chemical equation for the ionization of each acid and indicate whether it proceeds 100% to products or not.
- HNO3
- HNO2
- HI3
-
Write a chemical equation for the ionization of each base and indicate whether it proceeds 100% to products or not.
- NH3
- (CH3)3N
- Mg(OH)2
-
Write the balanced chemical equation for the reaction of each acid and base pair.
- HCl + C5H5N
- H2C2O4 + NH3
- HNO2 + C7H9N
-
Write the balanced chemical equation for the reaction of each acid and base pair.
- H3C5H5O7 + Mg(OH)2
- HC3H3O3 + (CH3)3N
- HBr + Fe(OH)3
-
Identify each salt as neutral, acidic, or basic.
- NaBr
- Fe(NO3)2
- Fe(NO3)3
-
Identify each salt as neutral, acidic, or basic.
- NH4I
- C2H5NH3Cl
- KI
-
Identify each salt as neutral, acidic, or basic.
- NaNO2
- NaNO3
- NH4NO3
-
Identify each salt as neutral, acidic, or basic.
- KC2H3O2
- KHSO4
- KClO3
-
Write the hydrolysis reaction that occurs, if any, when each salt dissolves in water.
- K2SO3
- KI
- NH4ClO3
-
Write the hydrolysis reaction that occurs, if any, when each salt dissolves in water.
- NaNO3
- CaC2O4
- C5H5NHCl
-
When NH4NO2 dissolves in H2O, both ions hydrolyze. Write chemical equations for both reactions. Can you tell if the solution will be acidic or basic overall?
-
When pyridinium acetate (C5H5NHC2H3O2) dissolves in H2O, both ions hydrolyze. Write chemical equations for both reactions. Can you tell if the solution will be acidic or basic overall?
-
A lab technician mixes a solution of 0.015 M Mg(OH)2. Is the resulting OH− concentration greater than, equal to, or less than 0.015 M? Explain your answer.
-
A lab technician mixes a solution of 0.55 M HNO3. Is the resulting H+ concentration greater than, equal to, or less than 0.55 M? Explain your answer.
Answers
-
A strong acid is 100% ionized in aqueous solution, whereas a weak acid is not 100% ionized.
-
-
- weak acid
- strong acid
- weak acid
-
-
- HNO3(aq) → H+(aq) + NO3−(aq); proceeds 100%
- HNO2(aq) → H+(aq) + NO2−(aq); does not proceed 100%
- HI3(aq) → H+(aq) + I3−(aq); does not proceed 100%
-
-
- HCl + C5H5N → Cl− + C5H5NH+
- H2C2O4 + 2NH3 → C2O42− + 2NH4+
- HNO2 + C7H9N → NO2− + C7H9NH+
-
-
- neutral
- acidic
- acidic
-
-
- basic
- neutral
- acidic
-
-
- SO32− + H2O → HSO3− + OH−
- no reaction
- NH4+ + H2O → NH3 + H3O+
-
-
NH4+ + H2O → NH3 + H3O+; NO2− + H2O → HNO2 + OH−; it is not possible to determine whether the solution will be acidic or basic.
-
-
greater than 0.015 M because there are two OH− ions per formula unit of Mg(OH)2
-
12.5 Autoionization of Water
Learning Objectives
- Describe the autoionization of water.
- Calculate the concentrations of H+ and OH− in solutions, knowing the other concentration.
We have already seen that H2O can act as an acid or a base:
NH3 + H2O → NH4+ + OH− (H2O acts as an acid) HCl + H2O → H3O+ + Cl− (H2O acts as a base)It may not surprise you to learn, then, that within any given sample of water, some H2O molecules are acting as acids, and other H2O molecules are acting as bases. The chemical equation is as follows:
H2O + H2O → H3O+ + OH−This occurs only to a very small degree: only about 6 in 108 H2O molecules are participating in this process, which is called the autoionization of waterWater molecules act as acids (proton donors) and bases (proton acceptors) with each other to a tiny extent in all aqueous solutions.. At this level, the concentration of both H+(aq) and OH−(aq) in a sample of pure H2O is about 1.0 × 10−7 M. If we use square brackets—[ ]—around a dissolved species to imply the molar concentration of that species, we have
[H+] = [OH−] = 1.0 × 10−7 Mfor any sample of pure water because H2O can act as both an acid and a base. The product of these two concentrations is 1.0 × 10−14:
[H+] × [OH−] = (1.0 × 10−7)(1.0 × 10−7) = 1.0 × 10−14In acids, the concentration of H+(aq)—[H+]—is greater than 1.0 × 10−7 M, while for bases the concentration of OH−(aq)—[OH−]—is greater than 1.0 × 10−7 M. However, the product of the two concentrations—[H+][OH−]—is always equal to 1.0 × 10−14, no matter whether the aqueous solution is an acid, a base, or neutral:
[H+][OH−] = 1.0 × 10−14This value of the product of concentrations is so important for aqueous solutions that it is called the autoionization constant of waterThe product of the hydrogen ion and hydroxide ion concentrations. and is denoted Kw:
Kw = [H+][OH−] = 1.0 × 10−14This means that if you know [H+] for a solution, you can calculate what [OH−] has to be for the product to equal 1.0 × 10−14, or if you know [OH−], you can calculate [H+]. This also implies that as one concentration goes up, the other must go down to compensate so that their product always equals the value of Kw.
Example 9
What is [OH−] of an aqueous solution if [H+] is 1.0 × 10−4 M?
Solution
Using the expression and known value for Kw,
Kw = [H+][OH−] = 1.0 × 10−14 = (1.0 × 10−4)[OH−]We solve by dividing both sides of the equation by 1.0 × 10−4:
It is assumed that the concentration unit is molarity, so [OH−] is 1.0 × 10−10 M.
Test Yourself
What is [H+] of an aqueous solution if [OH−] is 1.0 × 10−9 M?
Answer
1.0 × 10−5 M
When you have a solution of a particular acid or base, you need to look at the formula of the acid or base to determine the number of H+ or OH− ions in the formula unit because [H+] or [OH−] may not be the same as the concentration of the acid or base itself.
Example 10
What is [H+] in a 0.0044 M solution of Ca(OH)2?
Solution
We begin by determining [OH−]. The concentration of the solute is 0.0044 M, but because Ca(OH)2 is a strong base, there are two OH− ions in solution for every formula unit dissolved, so the actual [OH−] is two times this, or 2 × 0.0044 M = 0.0088 M. Now we can use the Kw expression:
[H+][OH−] = 1.0 × 10−14 = [H+](0.0088 M)Dividing both sides by 0.0088:
[H+] has decreased significantly in this basic solution.
Test Yourself
What is [OH−] in a 0.00032 M solution of H2SO4? (Hint: assume both H+ ions ionize.)
Answer
1.6 × 10−11 M
For strong acids and bases, [H+] and [OH−] can be determined directly from the concentration of the acid or base itself because these ions are 100% ionized by definition. However, for weak acids and bases, this is not so. The degree, or percentage, of ionization would need to be known before we can determine [H+] and [OH−].
Example 11
A 0.0788 M solution of HC2H3O2 is 3.0% ionized into H+ ions and C2H3O2− ions. What are [H+] and [OH−] for this solution?
Solution
Because the acid is only 3.0% ionized, we can determine [H+] from the concentration of the acid. Recall that 3.0% is 0.030 in decimal form:
[H+] = 0.030 × 0.0788 = 0.00236 MWith this [H+], then [OH−] can be calculated as follows:
This is about 30 times higher than would be expected for a strong acid of the same concentration.
Test Yourself
A 0.0222 M solution of pyridine (C5H5N) is 0.44% ionized into pyridinium ions (C5H5NH+) and OH− ions. What are [OH−] and [H+] for this solution?
Answer
[OH−] = 9.77 × 10−5 M; [H+] = 1.02 × 10−10 M
Key Takeaway
- In any aqueous solution, the product of [H+] and [OH−] equals 1.0 × 10−14.
Exercises
-
Does [H+] remain constant in all aqueous solutions? Why or why not?
-
Does [OH−] remain constant in all aqueous solutions? Why or why not?
-
What is the relationship between [H+] and Kw? Write a mathematical expression that relates them.
-
What is the relationship between [OH−] and Kw? Write a mathematical expression that relates them.
-
Write the chemical equation for the autoionization of water and label the conjugate acid-base pairs.
-
Write the reverse of the reaction for the autoionization of water. It is still an acid-base reaction? If so, label the acid and base.
-
For a given aqueous solution, if [H+] = 1.0 × 10−3 M, what is [OH−]?
-
For a given aqueous solution, if [H+] = 1.0 × 10−9 M, what is [OH−]?
-
For a given aqueous solution, if [H+] = 7.92 × 10−5 M, what is [OH−]?
-
For a given aqueous solution, if [H+] = 2.07 × 10−11 M, what is [H+]?
-
For a given aqueous solution, if [OH−] = 1.0 × 10−5 M, what is [H+]?
-
For a given aqueous solution, if [OH−] = 1.0 × 10−12 M, what is [H+]?
-
For a given aqueous solution, if [OH−] = 3.77 × 10−4 M, what is [H+]?
-
For a given aqueous solution, if [OH−] = 7.11 × 10−10 M, what is [H+]?
-
What are [H+] and [OH−] in a 0.344 M solution of HNO3?
-
What are [H+] and [OH−] in a 2.86 M solution of HBr?
-
What are [H+] and [OH−] in a 0.00338 M solution of KOH?
-
What are [H+] and [OH−] in a 6.02 × 10−4 M solution of Ca(OH)2?
-
If HNO2 is dissociated only to an extent of 0.445%, what are [H+] and [OH−] in a 0.307 M solution of HNO2?
-
If (C2H5)2NH is dissociated only to an extent of 0.077%, what are [H+] and [OH−] in a 0.0955 M solution of (C2H5)2NH?
Answers
-
[H+] varies with the amount of acid or base in a solution.
-
-
-
-
H2O + H2O → H3O+ + OH−; H2O/H3O+ and H2O/OH−
-
-
1.0 × 10−11 M
-
-
1.26 × 10−10 M
-
-
1.0 × 10−9 M
-
-
2.65 × 10−11 M
-
-
[H+] = 0.344 M; [OH−] = 2.91 × 10−14 M
-
-
[OH−] = 0.00338 M; [H+] = 2.96 × 10−12 M
-
-
[H+] = 0.00137 M; [OH−] = 7.32 × 10−12 M
-
12.6 The pH Scale
Learning Objectives
- Define pH.
- Determine the pH of acidic and basic solutions.
As we have seen, [H+] and [OH−] values can be markedly different from one aqueous solution to another. So chemists defined a new scale that succinctly indicates the concentrations of either of these two ions.
pHThe negative logarithm of the hydrogen ion concentration. is a logarithmic function of [H+]:
pH = −log[H+]pH is usually (but not always) between 0 and 14. Knowing the dependence of pH on [H+], we can summarize as follows:
- If pH < 7, then the solution is acidic.
- If pH = 7, then the solution is neutral.
- If pH > 7, then the solution is basic.
This is known as the pH scaleThe range of values from 0 to 14 that describes the acidity or basicity of a solution.. You can use pH to make a quick determination whether a given aqueous solution is acidic, basic, or neutral.
Example 12
Label each solution as acidic, basic, or neutral based only on the stated pH.
- milk of magnesia, pH = 10.5
- pure water, pH = 7
- wine, pH = 3.0
Solution
- With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia is largely Mg(OH)2.)
- Pure water, with a pH of 7, is neutral.
- With a pH of less than 7, wine is acidic.
Test Yourself
Identify each substance as acidic, basic, or neutral based only on the stated pH.
- human blood, pH = 7.4
- household ammonia, pH = 11.0
- cherries, pH = 3.6
Answers
- basic
- basic
- acidic
Table 12.3 "Typical pH Values of Various Substances*" gives the typical pH values of some common substances. Note that several food items are on the list, and most of them are acidic.
Table 12.3 Typical pH Values of Various Substances*
| Substance | pH |
|---|---|
| stomach acid | 1.7 |
| lemon juice | 2.2 |
| vinegar | 2.9 |
| soda | 3.0 |
| wine | 3.5 |
| coffee, black | 5.0 |
| milk | 6.9 |
| pure water | 7.0 |
| blood | 7.4 |
| seawater | 8.5 |
| milk of magnesia | 10.5 |
| ammonia solution | 12.5 |
| 1.0 M NaOH | 14.0 |
| *Actual values may vary depending on conditions. | |
pH is a logarithmic scale. A solution that has a pH of 1.0 has 10 times the [H+] as a solution with a pH of 2.0, which in turn has 10 times the [H+] as a solution with a pH of 3.0 and so forth.
Using the definition of pH, it is also possible to calculate [H+] (and [OH−]) from pH and vice versa. The general formula for determining [H+] from pH is as follows:
[H+] = 10−pHYou need to determine how to evaluate the above expression on your calculator. Ask your instructor if you have any questions. The other issue that concerns us here is significant figures. Because the number(s) before the decimal point in a logarithm relate to the power on 10, the number of digits after the decimal point is what determines the number of significant figures in the final answer:
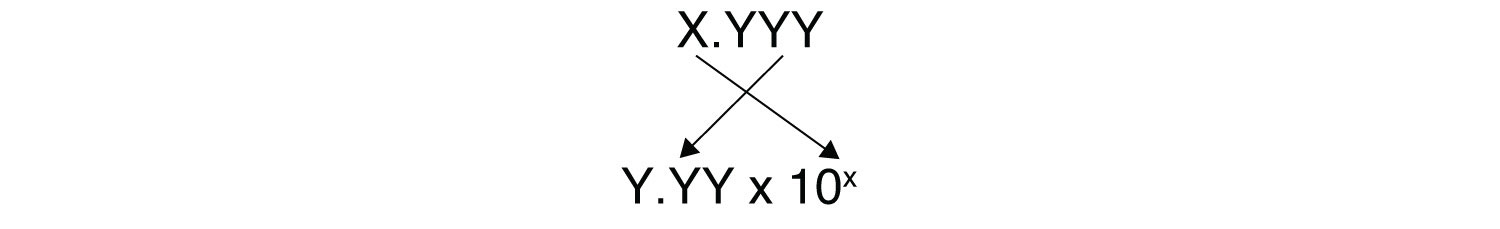
Example 13
What are [H+] and [OH−] for an aqueous solution whose pH is 4.88?
Solution
We need to evaluate the expression
[H+] = 10−4.88Depending on the calculator you use, the method for solving this problem will vary. In some cases, the “−4.88” is entered and a “10x” key is pressed; for other calculators, the sequence of keystrokes is reversed. In any case, the correct numerical answer is as follows:
[H+] = 1.3 × 10−5 MBecause 4.88 has two digits after the decimal point, [H+] is limited to two significant figures. From this, [OH−] can be determined:
Test Yourself
What are [H+] and [OH−] for an aqueous solution whose pH is 10.36?
Answer
[H+] = 4.4 × 10−11 M; [OH−] = 2.3 × 10−4 M
There is an easier way to relate [H+] and [OH−]. We can also define pOHThe negative logarithm of the hydroxide ion concentration. similar to pH:
pOH = −log[OH−](In fact, p“anything” is defined as the negative logarithm of that anything.) This also implies that
[OH−] = 10−pOHA simple and useful relationship is that for any aqueous solution,
pH + pOH = 14This relationship makes it simple to determine pH from pOH or pOH from pH and then calculate the resulting ion concentration.
Example 14
The pH of a solution is 8.22. What are pOH, [H+], and [OH−]?
Solution
Because the sum of pH and pOH equals 14, we have
8.22 + pOH = 14Subtracting 8.22 from 14, we get
pOH = 5.78Now we evaluate the following two expressions:
[H+] = 10−8.22 [OH−] = 10−5.78So
[H+] = 6.0 × 10−9 M [OH−] = 1.7 × 10−6 MTest Yourself
The pOH of a solution is 12.04. What are pH, [H+], and [OH−]?
Answer
pH = 1.96; [H+] = 1.1 × 10−2 M; [OH−] = 9.1 × 10−13 M
Key Takeaways
- pH is a logarithmic function of [H+].
- [H+] can be calculated directly from pH.
- pOH is related to pH and can be easily calculated from pH.
Exercises
-
Define pH. How is it related to pOH?
-
Define pOH. How is it related to pH?
-
What is the pH range for an acidic solution?
-
What is the pH range for a basic solution?
-
What is [H+] for a neutral solution?
-
What is [OH−] for a neutral solution? Compare your answer to Exercise 6. Does this make sense?
-
Which substances in Table 12.3 "Typical pH Values of Various Substances*" are acidic?
-
Which substances in Table 12.3 "Typical pH Values of Various Substances*" are basic?
-
What is the pH of a solution when [H+] is 3.44 × 10−4 M?
-
What is the pH of a solution when [H+] is 9.04 × 10−13 M?
-
What is the pH of a solution when [OH−] is 6.22 × 10−7 M?
-
What is the pH of a solution when [OH−] is 0.0222 M?
-
What is the pOH of a solution when [H+] is 3.44 × 10−4 M?
-
What is the pOH of a solution when [H+] is 9.04 × 10−13 M?
-
What is the pOH of a solution when [OH−] is 6.22 × 10−7 M?
-
What is the pOH of a solution when [OH−] is 0.0222 M?
-
If a solution has a pH of 0.77, what is its pOH, [H+], and [OH−]?
-
If a solution has a pOH of 13.09, what is its pH, [H+], and [OH−]?
Answers
-
pH is the negative logarithm of [H+] and is equal to 14 − pOH.
-
-
pH < 7
-
-
1.0 × 10−7 M
-
-
Every entry above pure water is acidic.
-
-
3.46
-
-
7.79
-
-
10.54
-
-
6.21
-
-
pOH = 13.23; [H+] = 1.70 × 10−1 M; [OH−] = 5.89 × 10−14 M
-
12.7 Buffers
Learning Objectives
- Define buffer.
- Correctly identify the two components of a buffer.
As indicated in Section 12.4 "Strong and Weak Acids and Bases and Their Salts", weak acids are relatively common, even in the foods we eat. But we occasionally encounter a strong acid or base, such as stomach acid, which has a strongly acidic pH of 1.7. By definition, strong acids and bases can produce a relatively large amount of H+ or OH− ions and consequently have marked chemical activities. In addition, very small amounts of strong acids and bases can change the pH of a solution very quickly. If 1 mL of stomach acid [approximated as 0.1 M HCl(aq)] were added to the bloodstream and no correcting mechanism were present, the pH of the blood would decrease from about 7.4 to about 4.7—a pH that is not conducive to continued living. Fortunately, the body has a mechanism for minimizing such dramatic pH changes.
The mechanism involves a bufferA solution that resists dramatic changes in pH., a solution that resists dramatic changes in pH. Buffers do so by being composed of certain pairs of solutes: either a weak acid plus a salt derived from that weak acid or a weak base plus a salt of that weak base. For example, a buffer can be composed of dissolved HC2H3O2 (a weak acid) and NaC2H3O2 (the salt derived from that weak acid). Another example of a buffer is a solution containing NH3 (a weak base) and NH4Cl (a salt derived from that weak base).
Let us use an HC2H3O2/NaC2H3O2 buffer to demonstrate how buffers work. If a strong base—a source of OH−(aq) ions—is added to the buffer solution, those OH− ions will react with the HC2H3O2 in an acid-base reaction:
HC2H3O2(aq) + OH−(aq) → H2O(ℓ) + C2H3O2−(aq)Rather than changing the pH dramatically by making the solution basic, the added OH− ions react to make H2O, so the pH does not change much.
If a strong acid—a source of H+ ions—is added to the buffer solution, the H+ ions will react with the anion from the salt. Because HC2H3O2 is a weak acid, it is not ionized much. This means that if lots of H+ ions and C2H3O2− ions are present in the same solution, they will come together to make HC2H3O2:
H+(aq) + C2H3O2−(aq) → HC2H3O2(aq)Rather than changing the pH dramatically and making the solution acidic, the added H+ ions react to make molecules of a weak acid. Figure 12.2 "The Actions of Buffers" illustrates both actions of a buffer.
Figure 12.2 The Actions of Buffers
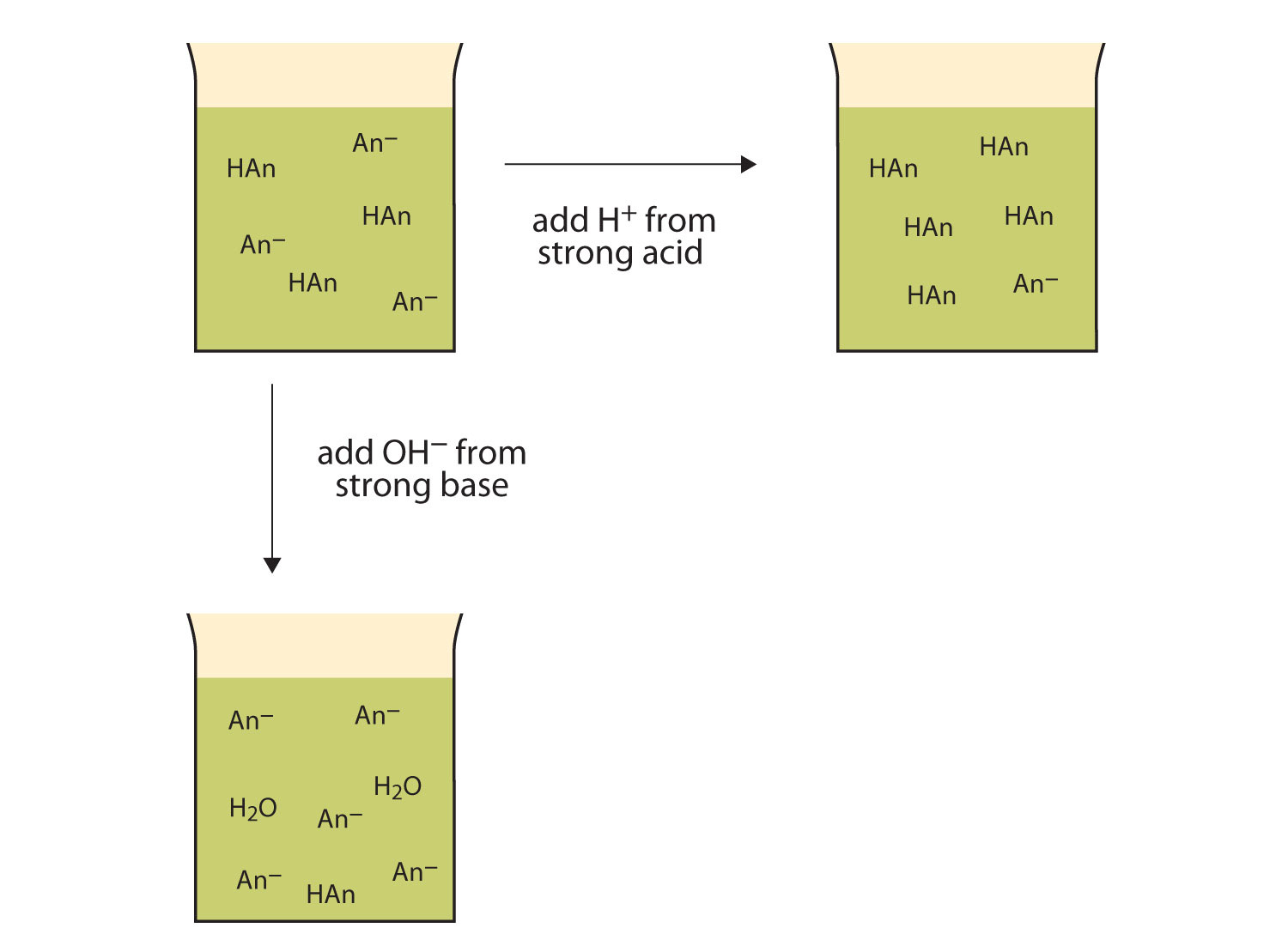
Buffers can react with both strong acids (top) and strong bases (side) to minimize large changes in pH.
Buffers made from weak bases and salts of weak bases act similarly. For example, in a buffer containing NH3 and NH4Cl, NH3 molecules can react with any excess H+ ions introduced by strong acids:
NH3(aq) + H+(aq) → NH4+(aq)while the NH4+(aq) ion can react with any OH− ions introduced by strong bases:
NH4+(aq) + OH−(aq) → NH3(aq) + H2O(ℓ)Example 15
Which combinations of compounds can make a buffer solution?
- HCHO2 and NaCHO2
- HCl and NaCl
- CH3NH2 and CH3NH3Cl
- NH3 and NaOH
Solution
- HCHO2 is formic acid, a weak acid, while NaCHO2 is the salt made from the anion of the weak acid (the formate ion [CHO2−]). The combination of these two solutes would make a buffer solution.
- HCl is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution.
- CH3NH2 is methylamine, which is like NH3 with one of its H atoms substituted with a CH3 group. Because it is not listed in Table 12.2 "Strong Acids and Bases", we can assume that it is a weak base. The compound CH3NH3Cl is a salt made from that weak base, so the combination of these two solutes would make a buffer solution.
- NH3 is a weak base, but NaOH is a strong base. The combination of these two solutes would not make a buffer solution.
Test Yourself
Which combinations of compounds can make a buffer solution?
- NaHCO3 and NaCl
- H3PO4 and NaH2PO4
- NH3 and (NH4)3PO4
- NaOH and NaCl
Answers
- no
- yes
- yes
- no
Buffers work well only for limited amounts of added strong acid or base. Once either solute is completely reacted, the solution is no longer a buffer, and rapid changes in pH may occur. We say that a buffer has a certain capacityThe amount of strong acid or base a buffer can counteract.. Buffers that have more solute dissolved in them to start with have larger capacities, as might be expected.
Human blood has a buffering system to minimize extreme changes in pH. One buffer in blood is based on the presence of HCO3− and H2CO3 [the second compound is another way to write CO2(aq)]. With this buffer present, even if some stomach acid were to find its way directly into the bloodstream, the change in the pH of blood would be minimal. Inside many of the body’s cells, there is a buffering system based on phosphate ions.
Food and Drink App: The Acid That Eases Pain
Although medicines are not exactly “food and drink,” we do ingest them, so let’s take a look at an acid that is probably the most common medicine: acetylsalicylic acid, also known as aspirin. Aspirin is well known as a pain reliever and antipyretic (fever reducer).
The structure of aspirin is shown in the accompanying figure. The acid part is circled; it is the H atom in that part that can be donated as aspirin acts as a Brønsted-Lowry acid. Because it is not given in Table 12.2 "Strong Acids and Bases", acetylsalicylic acid is a weak acid. However, it is still an acid, and given that some people consume relatively large amounts of aspirin daily, its acidic nature can cause problems in the stomach lining, despite the stomach’s defenses against its own stomach acid.
Figure 12.3 The Molecular Structure of Aspirin
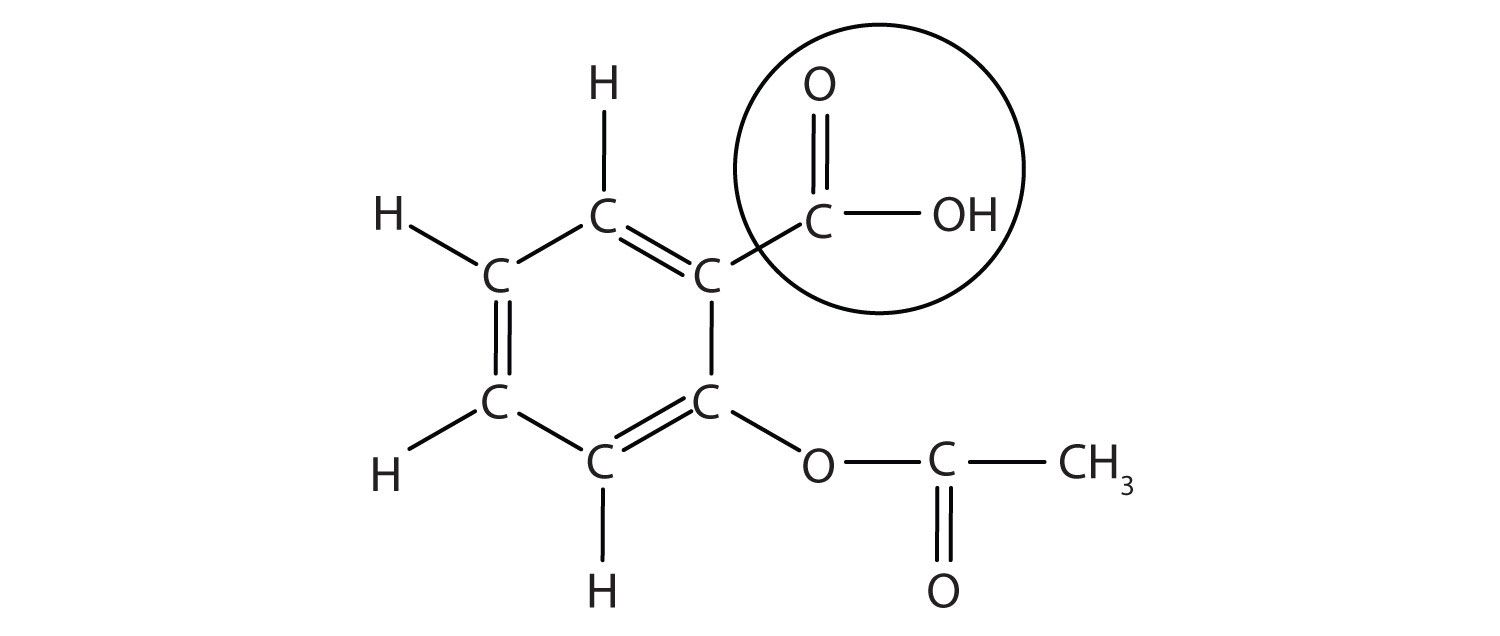
The circled atoms are the acid part of the molecule.
Because the acid properties of aspirin may be problematic, many aspirin brands offer a “buffered aspirin” form of the medicine. In these cases, the aspirin also contains a buffering agent—usually MgO—that regulates the acidity of the aspirin to minimize its acidic side effects.
As useful and common as aspirin is, it was formally marketed as a drug starting in 1899. The US Food and Drug Administration (FDA), the governmental agency charged with overseeing and approving drugs in the United States, wasn’t formed until 1906. Some have argued that if the FDA had been formed before aspirin was introduced, aspirin may never have gotten approval due to its potential for side effects—gastrointestinal bleeding, ringing in the ears, Reye’s syndrome (a liver problem), and some allergic reactions. However, recently aspirin has been touted for its effects in lessening heart attacks and strokes, so it is likely that aspirin is here to stay.
Key Takeaway
- A buffer is a solution that resists sudden changes in pH.
Exercises
-
Define buffer. What two related chemical components are required to make a buffer?
-
Can a buffer be made by combining a strong acid with a strong base? Why or why not?
-
Which combinations of compounds can make a buffer? Assume aqueous solutions.
- HCl and NaCl
- HNO2 and NaNO2
- NH4NO3 and HNO3
- NH4NO3 and NH3
-
Which combinations of compounds can make a buffer? Assume aqueous solutions.
- H3PO4 and Na3PO4
- NaHCO3 and Na2CO3
- NaNO3 and Ca(NO3)2
- HN3 and NH3
-
For each combination in Exercise 3 that is a buffer, write the chemical equations for the reactions of the buffer components when a strong acid and a strong base is added.
-
For each combination in Exercise 4 that is a buffer, write the chemical equations for the reactions of the buffer components when a strong acid and a strong base is added.
-
The complete phosphate buffer system is based on four substances: H3PO4, H2PO4−, HPO42−, and PO43−. What different buffer solutions can be made from these substances?
-
Explain why NaBr cannot be a component in either an acidic or a basic buffer.
-
Two solutions are made containing the same concentrations of solutes. One solution is composed of H3PO4 and Na3PO4, while the other is composed of HCN and NaCN. Which solution should have the larger capacity as a buffer?
-
Two solutions are made containing the same concentrations of solutes. One solution is composed of NH3 and NH4NO3, while the other is composed of H2SO4 and Na2SO4. Which solution should have the larger capacity as a buffer?
Answers
-
A buffer is the combination of a weak acid or base and a salt of that weak acid or base.
-
-
- no
- yes
- no
- yes
-
-
3b: strong acid: NO2− + H+ → HNO2; strong base: HNO2 + OH− → NO2− + H2O; 3d: strong base: NH4+ + OH− → NH3 + H2O; strong acid: NH3 + H+ → NH4+
-
-
Buffers can be made from three combinations: (1) H3PO4 and H2PO4−, (2) H2PO4− and HPO42−, and (3) HPO42− and PO43−. (Technically, a buffer can be made from any two components.)
-
-
The phosphate buffer should have the larger capacity.
-
12.8 End-of-Chapter Material
Additional Exercises
-
Write the balanced chemical equation between Zn metal and HCl(aq). The other product is ZnCl2.
-
Write the neutralization reaction in which ZnCl2, also found in Exercise 1, is the salt product.
-
Why isn’t an oxide compound like CaO considered a salt? (Hint: what acid-base combination would be needed to make it if it were a salt?)
-
Metal oxides are considered basic because they react with H2O to form OH compounds. Write the chemical equation for a reaction that forms a base when CaO is combined with H2O.
-
Write the balanced chemical equation between aluminum hydroxide and sulfuric acid.
-
Write the balanced chemical equation between phosphoric acid and barium hydroxide.
-
Write the equation for the chemical reaction that occurs when caffeine (C8H10N4O2) acts as a Brønsted-Lowry base.
-
Citric acid (C6H8O7) is the acid found in citrus fruits. It can lose a maximum of three H+ ions in the presence of a base. Write the chemical equations for citric acid acting stepwise as a Brønsted-Lowry acid.
-
Can an amphiprotic substance be a strong acid and a strong base at the same time? Explain your answer.
-
Can an amphiprotic substance be a weak acid and a weak base at the same time? If so, explain why and give an example.
-
Under what conditions will the equivalence point of a titration be slightly acidic?
-
Under what conditions will the equivalence point of a titration be slightly basic?
-
Write the chemical equation for the autoionization of NH3.
-
Write the chemical equation for the autoionization of HF.
-
What is the pOH range for an acidic solution?
-
What is the pOH range for a basic solution?
-
The concentration of commercial HCl is about 12 M. What is its pH and pOH?
-
The concentration of concentrated H2SO4 is about 18 M. Assuming only one H+ comes off the H2SO4 molecule, what is its pH and pOH? What would the pH and pOH be if the second H+ were also ionized?
Answers
-
Zn + 2HCl → ZnCl2 + H2
-
-
The O2− ion would come from H2O, which is not considered a classic acid in the Arrhenius sense.
-
-
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
-
-
C8H10N4O2 + H2O → C8H10N4O2H+ + OH−; the H+ ion attaches to one of the N atoms in the caffeine molecule.
-
-
As a strong acid or base, an amphiprotic substance reacts 100% as an acid or a base, so it cannot be a base or an acid at the same time.
-
-
if the salt produced is an acidic salt
-
-
NH3 + NH3 → NH4+ + NH2−
-
-
pOH > 7
-
-
pH = −1.08; pOH = 15.08
-




