This is “The Elements of Group 16 (The Chalcogens)”, section 22.4 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there. To download a .zip file containing this book to use offline, simply click here.
22.4 The Elements of Group 16 (The Chalcogens)
Learning Objective
- To understand the trends in properties and reactivity of the group 16 elements: the chalcogens.
The chalcogens are the first group in the p block to have no stable metallic elements. All isotopes of polonium (Po), the only metal in group 16, are radioactive, and only one element in the group, tellurium (Te), can even be described as a semimetal. As in groups 14 and 15, the lightest element of group 16, oxygen, is found in nature as the free element.
Of the group 16 elements, only sulfur was known in ancient times; the others were not discovered until the late 18th and 19th centuries. Sulfur is frequently found as yellow crystalline deposits of essentially pure S8 in areas of intense volcanic activity or around hot springs. As early as the 15th century BC, sulfur was used as a fumigant in Homeric Greece because, when burned, it produces SO2 fumes that are toxic to most organisms, including vermin hiding in the walls and under the floors of houses. Hence references to sulfur are common in ancient literature, frequently in the context of religious purification. In fact, the association of sulfur with the divine was so pervasive that the prefixes thio- (meaning “sulfur”) and theo- (meaning “god”) have the same root in ancient Greek. Though used primarily in the production of sulfuric acid, sulfur is also used to manufacture gunpowder and as a cross-linking agent for rubber, which enables rubber to hold its shape but retain its flexibility.
Note the Pattern
Group 16 is the first group in the p block with no stable metallic elements.
Oxygen was not discovered until 1771, when the Swedish pharmacist Carl Wilhelm Scheele found that heating compounds such as KNO3, Ag2CO3, and HgO produced a colorless, odorless gas that supported combustion better than air. The results were not published immediately, however, so Scheele’s work remained unknown until 1777. Unfortunately, this was nearly two years after a paper by the English chemist Joseph Priestley had been published, describing the isolation of the same gas by using a magnifying glass to focus the sun’s rays on a sample of HgO. Oxygen is used primarily in the steel industry during the conversion of crude iron to steel using the Bessemer process. (For more information on the Bessemer process, see Chapter 23 "The ".) Another important industrial use of oxygen is in the production of TiO2, which is commonly used as a white pigment in paints, paper, and plastics.

A crystalline sulfur deposit. This sulfur deposit is located around a volcanic vent in Kilauea Crater, Hawaii.
Tellurium was discovered accidentally in 1782 by the Austrian chemist Franz Joseph Müller von Reichenstein, the chief surveyor of mines in Transylvania who was also responsible for the analysis of ore samples. The silvery-white metal had the same density as antimony but very different properties. Because it was difficult to analyze, Müller called it metallum problematicum (meaning “difficult metal”). The name tellurium (from the Latin tellus, meaning “earth”) was coined by another Austrian chemist, Martin Klaproth, who demonstrated in 1798 that Müller’s “difficult metal” was actually a new element. Tellurium is used to color glass and ceramics, in the manufacture of blasting caps, and in thermoelectric devices.
Selenium (Se) was first isolated in 1817 by the Swedish chemist Jöns Jakob Berzelius, who also discovered silicon. He had invested money in a sulfuric acid plant and decided to investigate a foul-smelling contaminant that formed a red precipitate. Although he initially thought the contaminant was tellurium, further study showed that it was actually a new element similar to tellurium. To emphasize the similarities, Berzelius named the new element selenium (after the Greek selene, meaning “moon”). Selenium is used primarily as a minor ingredient to decolorize glass. Because it is photosensitive, selenium is also used to capture images in the photocopying process (Figure 22.12 "The Chemistry of Photocopying").
Jöns Jakob Berzelius (1779–1848)
Berzelius was born into a well-educated Swedish family, but both parents died when he was young. He studied medicine at the University of Uppsala, where his experiments with electroshock therapy caused his interests to turn to electrochemistry. Berzelius devised the system of chemical notation that we use today. In addition, he discovered six elements (cerium, thorium, selenium, silicon, titanium, and zirconium).
Figure 22.12 The Chemistry of Photocopying

Because amorphous selenium is a photosensitive semiconductor, exposing an electrostatically charged Se film to light causes the positive charge on the film to be discharged in all areas that are white in the original. Dark areas in the original block the light and generate an invisible, positively charged image. To produce an image on paper, negatively charged toner particles are attracted to the positive image, transferred to a negatively charged sheet of blank paper, and fused with the paper at high temperature to give a permanent image.
The heaviest chalcogen, polonium, was isolated after an extraordinary effort by Marie Curie. (For more information on radioactivity and polonium, see Chapter 1 "Introduction to Chemistry", Section 1.5 "The Atom".) Although she was never able to obtain macroscopic quantities of the element, which she named for her native country of Poland, she demonstrated that its chemistry required it to be assigned to group 16. Marie Curie was awarded a second Nobel Prize in Chemistry in 1911 for the discovery of radium and polonium.
Preparation and General Properties of the Group 16 Elements
Oxygen is by far the most abundant element in Earth’s crust and in the hydrosphere (about 44% and 86% by mass, respectively). The same process that is used to obtain nitrogen from the atmosphere produces pure oxygen. Oxygen can also be obtained by the electrolysis of water, the decomposition of alkali metal or alkaline earth peroxides or superoxides, or the thermal decomposition of simple inorganic salts, such as potassium chlorate in the presence of a catalytic amount of MnO2:
Equation 22.33
(For more information on electrolysis, see Chapter 19 "Electrochemistry". For more information on the alkali metals and the alkaline earth metals, see Chapter 21 "Periodic Trends and the ".)
Unlike oxygen, sulfur is not very abundant, but it is found as elemental sulfur in rock formations overlying salt domes, which often accompany petroleum deposits (Figure 2.22 "Top 25 Chemicals Produced in the United States in 2002*"). Sulfur is also recovered from H2S and organosulfur compounds in crude oil and coal and from metal sulfide ores such as pyrite (FeS2).

Pyrite (FeS2). Because of its lustrous golden yellow cubic crystals, FeS2 is sometimes mistaken for gold, giving rise to its common name “fool’s gold.” Real gold, however, is much denser than FeS2, and gold is soft and malleable rather than hard and brittle.
Because selenium and tellurium are chemically similar to sulfur, they are usually found as minor contaminants in metal sulfide ores and are typically recovered as by-products. Even so, they are as abundant in Earth’s crust as silver, palladium, and gold. One of the best sources of selenium and tellurium is the “slime” deposited during the electrolytic purification of copper. Both of these elements are notorious for the vile odors of many of their compounds. For example, when the body absorbs even trace amounts of tellurium, dimethyltellurium [(CH3)2Te] is produced and slowly released in the breath and perspiration, resulting in an intense garlic-like smell that is commonly called “tellurium breath.”
With their ns2np4 electron configurations, the chalcogens are two electrons short of a filled valence shell. Thus in reactions with metals, they tend to acquire two additional electrons to form compounds in the −2 oxidation state. This tendency is greatest for oxygen, the chalcogen with the highest electronegativity. The heavier, less electronegative chalcogens can lose either four np electrons or four np and two ns electrons to form compounds in the +4 and +6 oxidation state, respectively, as shown in Table 22.4 "Selected Properties of the Group 16 Elements". As with the other groups, the lightest member in the group, in this case oxygen, differs greatly from the others in size, ionization energy, electronegativity, and electron affinity, so its chemistry is unique. Also as in the other groups, the second and third members (sulfur and selenium) have similar properties because of shielding effects. Only polonium is metallic, forming either the hydrated Po2+ or Po4+ ion in aqueous solution, depending on conditions.
Table 22.4 Selected Properties of the Group 16 Elements
| Property | Oxygen | Sulfur | Selenium | Tellurium | Polonium |
|---|---|---|---|---|---|
| atomic symbol | O | S | Se | Te | Po |
| atomic number | 8 | 16 | 34 | 52 | 84 |
| atomic mass (amu) | 16.00 | 32.07 | 78.96 | 127.60 | 209 |
| valence electron configuration* | 2s22p4 | 3s23p4 | 4s24p4 | 5s25p4 | 6s26p4 |
| melting point/boiling point (°C) | −219/−183 | 115/445 | 221/685 | 450/988 | 254/962 |
| density (g/cm3) at 25°C | 1.31 | 2.07 | 4.81 | 6.24 | 9.20 |
| atomic radius (pm) | 48 | 88 | 103 | 123 | 135 |
| first ionization energy (kJ/mol) | 1314 | 1000 | 941 | 869 | 812 |
| normal oxidation state(s) | −2 | +6, +4, −2 | +6, +4, −2 | +6, +4, −2 | +2 (+4) |
| ionic radius (pm)† | 140 (−2) | 184 (−2), 29 (+6) | 198 (−2), 42 (+6) | 221 (−2), 56 (+6) | 230 (−2), 97 (+4) |
| electron affinity (kJ/mol) | −141 | −200 | −195 | −190 | −180 |
| electronegativity | 3.4 | 2.6 | 2.6 | 2.1 | 2.0 |
| standard reduction potential (E°, V) (E0 → H2E in acidic solution) | +1.23 | +0.14 | −0.40 | −0.79 | −1.00 |
| product of reaction with O2 | — | SO2 | SeO2 | TeO2 | PoO2 |
| type of oxide | — | acidic | acidic | amphoteric | basic |
| product of reaction with N2 | NO, NO2 | none | none | none | none |
| product of reaction with X2 | O2F2 | SF6, S2Cl2, S2Br2 | SeF6, SeX4 | TeF6, TeX4 | PoF4, PoCl2, PoBr2 |
| product of reaction with H2 | H2O | H2S | H2Se | none | none |
| *The configuration shown does not include filled d and f subshells. | |||||
| †The values cited for the hexacations are for six-coordinate ions and are only estimated values. | |||||
Reactions and Compounds of Oxygen
As in groups 14 and 15, the lightest group 16 member has the greatest tendency to form multiple bonds. Thus elemental oxygen is found in nature as a diatomic gas that contains a net double bond: O=O. As with nitrogen, electrostatic repulsion between lone pairs of electrons on adjacent atoms prevents oxygen from forming stable catenated compounds. In fact, except for O2, all compounds that contain O–O bonds are potentially explosive. Ozone, peroxides, and superoxides are all potentially dangerous in pure form. Ozone (O3), one of the most powerful oxidants known, is used to purify drinking water because it does not produce the characteristic taste associated with chlorinated water. Hydrogen peroxide (H2O2) is so thermodynamically unstable that it has a tendency to undergo explosive decomposition when impure:
Equation 22.34
Note the Pattern
As in groups 14 and 15, the lightest element in group 16 has the greatest tendency to form multiple bonds.
Despite the strength of the O=O bond ( = 494 kJ/mol), O2 is extremely reactive, reacting directly with nearly all other elements except the noble gases. Some properties of O2 and related species, such as the peroxide and superoxide ions, are in Table 22.5 "Some Properties of O". With few exceptions, the chemistry of oxygen is restricted to negative oxidation states because of its high electronegativity (χ = 3.4). Unlike the other chalcogens, oxygen does not form compounds in the +4 or +6 oxidation state. Oxygen is second only to fluorine in its ability to stabilize high oxidation states of metals in both ionic and covalent compounds. For example, AgO is a stable solid that contains silver in the unusual Ag(II) state, whereas OsO4 is a volatile solid that contains Os(VIII). Because oxygen is so electronegative, the O–H bond is highly polar, creating a large bond dipole moment that makes hydrogen bonding much more important for compounds of oxygen than for similar compounds of the other chalcogens.
Table 22.5 Some Properties of O2 and Related Diatomic Species
| Species | Bond Order | Number of Unpaired e− | O–O Distance (pm)* |
|---|---|---|---|
| O2+ | 2.5 | 1 | 112 |
| O2 | 2 | 2 | 121 |
| O2− | 1.5 | 1 | 133 |
| O22− | 1 | 0 | 149 |
| *Source of data: Lauri Vaska, “Dioxygen-Metal Complexes: Toward a Unified View,” Accounts of Chemical Research 9 (1976): 175. | |||
Metal oxides are usually basic, and nonmetal oxides are acidic, whereas oxides of elements that lie on or near the diagonal band of semimetals are generally amphoteric. A few oxides, such as CO and PbO2, are neutral and do not react with water, aqueous acid, or aqueous base. Nonmetal oxides are typically covalent compounds in which the bonds between oxygen and the nonmetal are polarized (Eδ+–Oδ−). Consequently, a lone pair of electrons on a water molecule can attack the partially positively charged E atom to eventually form an oxoacid. An example is reacting sulfur trioxide with water to form sulfuric acid:
Equation 22.35
H2O(l) + SO3(g) → H2SO4(aq)The oxides of the semimetals and of elements such as Al that lie near the metal/nonmetal dividing line are amphoteric, as we expect:
Equation 22.36
Al2O3(s) + 6H+(aq) → 2Al3+(aq) + 3H2O(l)Equation 22.37
Al2O3(s) + 2OH−(aq) + 3H2O(l) → 2Al(OH)4−(aq)Note the Pattern
Oxides of metals tend to be basic, oxides of nonmetals tend to be acidic, and oxides of elements in or near the diagonal band of semimetals are generally amphoteric.
Example 7
For each reaction, explain why the given products form.
- Ga2O3(s) + 2OH−(aq) + 3H2O(l) → 2Ga(OH)4−(aq)
- 3H2O2(aq) + 2MnO4−(aq) + 2H+(aq) → 3O2(g) + 2MnO2(s) + 4H2O(l)
- KNO3(s) KNO(s) + O2(g)
Given: balanced chemical equations
Asked for: why the given products form
Strategy:
Classify the type of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the observed reaction products form.
Solution:
- Gallium is a metal. We expect the oxides of metallic elements to be basic and therefore not to react with aqueous base. A close look at the periodic table, however, shows that gallium is close to the diagonal line of semimetals. Moreover, aluminum, the element immediately above gallium in group 13, is amphoteric. Consequently, we predict that gallium will behave like aluminum (Equation 22.37).
- Hydrogen peroxide is an oxidant that can accept two electrons per molecule to give two molecules of water. With a strong oxidant, however, H2O2 can also act as a reductant, losing two electrons (and two protons) to produce O2. Because the other reactant is permanganate, which is a potent oxidant, the only possible reaction is a redox reaction in which permanganate is the oxidant and hydrogen peroxide is the reductant. Recall that reducing permanganate often gives MnO2, an insoluble brown solid. Reducing MnO4− to MnO2 is a three-electron reduction, whereas the oxidation of H2O2 to O2 is a two-electron oxidation.
- This is a thermal decomposition reaction. Because KNO3 contains nitrogen in its highest oxidation state (+5) and oxygen in its lowest oxidation state (−2), a redox reaction is likely. Oxidation of the oxygen in nitrate to atomic oxygen is a two-electron process per oxygen atom. Nitrogen is likely to accept two electrons because oxoanions of nitrogen are known only in the +5 (NO3−) and +3 (NO2−) oxidation states.
Exercise
Predict the product(s) of each reaction and write a balanced chemical equation for each reaction.
- SiO2(s) + H+(aq) →
- NO(g) + O2(g) →
- SO3(g) + H2O(l) →
- H2O2(aq) + I–(aq) →
Answer:
- SiO2(s) + H+(aq) → no reaction
- 2NO(g) + O2(g) → 2NO2(g)
- SO3(g) + H2O(l) → H2SO4(aq)
- H2O2(aq) + 2I−(aq) → I2(aq) + 2OH−(aq)
Reactions and Compounds of the Heavier Chalcogens
Because most of the heavier chalcogens (group 16) and pnicogens (group 15) are nonmetals, they often form similar compounds. For example, both third-period elements of these groups (phosphorus and sulfur) form catenated compounds and form multiple allotropes. Consistent with periodic trends, the tendency to catenate decreases as we go down the column.
Sulfur and selenium both form a fairly extensive series of catenated species. For example, elemental sulfur forms S8 rings packed together in a complex “crankshaft” arrangement (Figure 18.15 "Two Forms of Elemental Sulfur and a Thermodynamic Cycle Showing the Transition from One to the Other"), and molten sulfur contains long chains of sulfur atoms connected by S–S bonds. Moreover, both sulfur and selenium form polysulfides (Sn2−) and polyselenides (Sen2−), with n ≤ 6. The only stable allotrope of tellurium is a silvery white substance whose properties and structure are similar to those of one of the selenium allotropes. Polonium, in contrast, shows no tendency to form catenated compounds. The striking decrease in structural complexity from sulfur to polonium is consistent with the decrease in the strength of single bonds and the increase in metallic character as we go down the group.
As in group 15, the reactivity of elements in group 16 decreases from lightest to heaviest. For example, selenium and tellurium react with most elements but not as readily as sulfur does. As expected for nonmetals, sulfur, selenium, and tellurium do not react with water, aqueous acid, or aqueous base, but all dissolve in strongly oxidizing acids such as HNO3 to form oxoacids such as H2SO4. In contrast to the other chalcogens, polonium behaves like a metal, dissolving in dilute HCl to form solutions that contain the Po2+ ion.
Note the Pattern
Just as with the other groups, the tendency to catenate, the strength of single bonds, and reactivity decrease down the group.
Fluorine reacts directly with all chalcogens except oxygen to produce the hexafluorides (YF6), which are extraordinarily stable and unreactive compounds. Four additional stable fluorides of sulfur are known; thus sulfur oxidation states range from +1 to +6 (Figure 22.13 "The Structures of the Known Fluorides of Sulfur"). In contrast, only four fluorides of selenium (SeF6, SeF4, FSeSeF, and SeSeF2) and only three of tellurium (TeF4, TeF6, and Te2F10) are known.
Figure 22.13 The Structures of the Known Fluorides of Sulfur
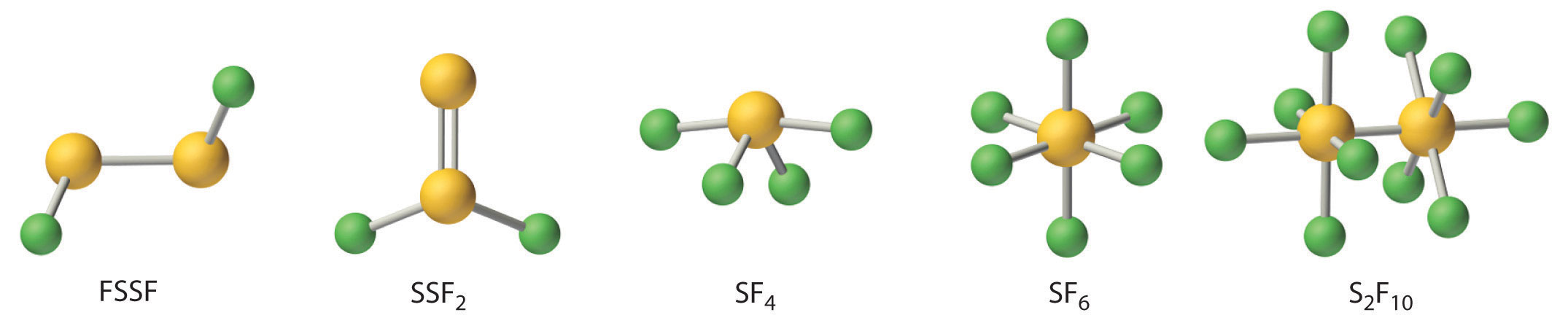
Five stable sulfur fluorides are known, containing sulfur in oxidation states ranging from +1 to +6. All are volatile molecular compounds that vary tremendously in stability and toxicity. Although both SF6 and S2F10 are very stable, S2F10 is toxic and SF6 is not. The other three are highly reactive substances.
Direct reaction of the heavier chalcogens with oxygen at elevated temperatures gives the dioxides (YO2), which exhibit a dramatic range of structures and properties. The dioxides become increasingly metallic in character down the group, as expected, and the coordination number of the chalcogen steadily increases. Thus SO2 is a gas that contains V-shaped molecules (as predicted by the valence-shell electron-pair repulsion model), SeO2 is a white solid with an infinite chain structure (each Se is three coordinate), TeO2 is a light yellow solid with a network structure in which each Te atom is four coordinate, and PoO2 is a yellow ionic solid in which each Po4+ ion is eight coordinate.
The dioxides of sulfur, selenium, and tellurium react with water to produce the weak, diprotic oxoacids (H2YO3—sulfurous, selenous, and tellurous acid, respectively). Both sulfuric acid and selenic acid (H2SeO4) are strong acids, but telluric acid [Te(OH)6] is quite different. Because tellurium is larger than either sulfur or selenium, it forms weaker π bonds to oxygen. As a result, the most stable structure for telluric acid is Te(OH)6, with six Te–OH bonds rather than Te=O bonds. Telluric acid therefore behaves like a weak triprotic acid in aqueous solution, successively losing the hydrogen atoms bound to three of the oxygen atoms. As expected for compounds that contain elements in their highest accessible oxidation state (+6 in this case), sulfuric, selenic, and telluric acids are oxidants. Because the stability of the highest oxidation state decreases with increasing atomic number, telluric acid is a stronger oxidant than sulfuric acid.
Note the Pattern
The stability of the highest oxidation state of the chalcogens decreases down the column.
Sulfur and, to a lesser extent, selenium react with carbon to form an extensive series of compounds that are structurally similar to their oxygen analogues. For example, CS2 and CSe2 are both volatile liquids that contain C=S or C=Se bonds and have the same linear structure as CO2. Because these double bonds are significantly weaker than the C=O bond, however, CS2, CSe2, and related compounds are less stable and more reactive than their oxygen analogues. The chalcogens also react directly with nearly all metals to form compounds with a wide range of stoichiometries and a variety of structures. Metal chalcogenides can contain either the simple chalcogenide ion (Y2−), as in Na2S and FeS, or polychalcogenide ions (Yn2−), as in FeS2 and Na2S5.
Note the Pattern
The dioxides of the group 16 elements become increasingly basic, and the coordination number of the chalcogen steadily increases down the group.
Ionic chalcogenides like Na2S react with aqueous acid to produce binary hydrides such as hydrogen sulfide (H2S). Because the strength of the Y–H bond decreases with increasing atomic radius, the stability of the binary hydrides decreases rapidly down the group. It is perhaps surprising that hydrogen sulfide, with its familiar rotten-egg smell, is much more toxic than hydrogen cyanide (HCN), the gas used to execute prisoners in the “gas chamber.” Hydrogen sulfide at relatively low concentrations deadens the olfactory receptors in the nose, which allows it to reach toxic levels without detection and makes it especially dangerous.
Example 8
For each reaction, explain why the given product forms or no reaction occurs.
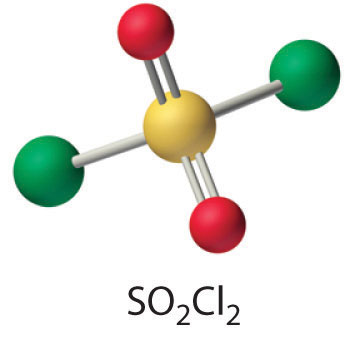
- SO2(g) + Cl2(g) → SO2Cl2(l)
- SF6(g) + H2O(l) → no reaction
- 2Se(s) + Cl2(g) → Se2Cl2(l)
Given: balanced chemical equations
Asked for: why the given products (or no products) form
Strategy:
Classify the type of reaction. Using periodic trends in atomic properties, thermodynamics, and kinetics, explain why the reaction products form or why no reaction occurs.
Solution:
-
One of the reactants (Cl2) is an oxidant. If the other reactant can be oxidized, then a redox reaction is likely. Sulfur dioxide contains sulfur in the +4 oxidation state, which is 2 less than its maximum oxidation state. Sulfur dioxide is also known to be a mild reducing agent in aqueous solution, producing sulfuric acid as the oxidation product. Hence a redox reaction is probable. The simplest reaction is the formation of SO2Cl2 (sulfuryl chloride), which is a tetrahedral species with two S–Cl and two S=O bonds.
- Sulfur hexafluoride is a nonmetallic halide. Such compounds normally react vigorously with water to produce an oxoacid of the nonmetal and the corresponding hydrohalic acid. In this case, however, we have a highly stable species, presumably because all of sulfur’s available orbitals are bonding orbitals. Thus SF6 is not likely to react with water.
- Here we have the reaction of a chalcogen with a halogen. The halogen is a good oxidant, so we can anticipate that a redox reaction will occur. Only fluorine is capable of oxidizing the chalcogens to a +6 oxidation state, so we must decide between SeCl4 and Se2Cl2 as the product. The stoichiometry of the reaction determines which of the two is obtained: SeCl4 or Se2Cl2.
Exercise
Predict the products of each reaction and write a balanced chemical equation for each reaction.
- Te(s) + Na(s)
- SF4(g) + H2O(l) →
- CH3SeSeCH3(soln) + K(s) →
- Li2Se(s) + H+(aq) →
Answer:
- Te(s) + 2Na(s) → Na2Te(s)
- SF4(g) + 3H2O(l) → H2SO3(aq) + 4HF(aq)
- CH3SeSeCH3(soln) + 2K(s) → 2KCH3Se(soln)
- Li2Se(s) + 2H+(aq) → H2Se(g) + 2Li+(aq)
Summary
Because the electronegativity of the chalcogens decreases down the group, so does their tendency to acquire two electrons to form compounds in the −2 oxidation state. The lightest member, oxygen, has the greatest tendency to form multiple bonds with other elements. It does not form stable catenated compounds, however, due to repulsions between lone pairs of electrons on adjacent atoms. Because of its high electronegativity, the chemistry of oxygen is generally restricted to compounds in which it has a negative oxidation state, and its bonds to other elements tend to be highly polar. Metal oxides are usually basic, and nonmetal oxides are acidic, whereas oxides of elements along the dividing line between metals and nonmetals are amphoteric. The reactivity, the strength of multiple bonds to oxygen, and the tendency to form catenated compounds all decrease down the group, whereas the maximum coordination numbers increase. Because Te=O bonds are comparatively weak, the most stable oxoacid of tellurium contains six Te–OH bonds. The stability of the highest oxidation state (+6) decreases down the group. Double bonds between S or Se and second-row atoms are weaker than the analogous C=O bonds because of reduced orbital overlap. The stability of the binary hydrides decreases down the group.
Key Takeaways
- The chalcogens have no stable metallic elements.
- The tendency to catenate, the strength of single bonds, and the reactivity all decrease moving down the group.
Conceptual Problems
-
Unlike the other chalcogens, oxygen does not form compounds in the +4 or +6 oxidation state. Why?
-
Classify each oxide as basic, acidic, amphoteric, or neutral.
- CaO
- SO2
- NO
- Rb2O
- PbO2
-
Classify each oxide as basic, acidic, amphoteric, or neutral.
- BaO
- Br2O
- SnO
- B2O3
- Sb2O3
-
Polarization of an oxide affects its solubility in acids or bases. Based on this, do you expect RuO2 to be an acidic, a basic, or a neutral oxide? Is the compound covalent? Justify your answers.
-
Arrange CrO3, Al2O3, Sc2O3, and BaO in order of increasing basicity.
-
As the atomic number of the group 16 elements increases, the complexity of their allotropes decreases. What factors account for this trend? Which chalcogen do you expect to polymerize the most readily? Why?
-
Arrange H3BO3, HIO4, and HNO2 in order of increasing acid strength.
-
Of OF2, SO2, P4O6, SiO2, and Al2O3, which is most ionic?
-
Of CO2, NO2, O2, SO2, Cl2O, H2O, NH3, and CH4, which do you expect to have the
- most polar covalent bond(s)?
- least polar covalent bond(s)?
-
Of Na2O2, MgO, Al2O3, and SiO2, which is most acidic?
-
Give an example of
- a covalent hydride that engages in strong hydrogen bonding.
- an amphoteric oxide.
-
The Si–O bond is shorter and stronger than expected. What orbitals are used in this bond? Do you expect Si to interact with Br in the same way? Why or why not?
Answers
-
Oxygen has the second highest electronegativity of any element; consequently, it prefers to share or accept electrons from other elements. Only with fluorine does oxygen form compounds in positive oxidation states.
-
-
- basic
- acidic
- amphoteric
- acidic
- amphoteric
-
-
CrO3 < Al2O3 < Sc2O3 < BaO
-
-
H3BO3 < HNO2 < HIO4
-
-
Most polar: H2O; least polar: O2
-
-
- H2O, HF, or NH3
- SnO or Al2O3
-
Structure and Reactivity
-
Considering its position in the periodic table, predict the following properties of selenium:
- chemical formulas of its most common oxide, most common chloride, and most common hydride
- solubility of its hydride in water, and the acidity or basicity of the resulting solution
- the principal ion formed in aqueous solution
-
Using arguments based on electronegativity, explain why ZnO is amphoteric. What product would you expect when ZnO reacts with an aqueous
- acid?
- base?
-
Write a balanced chemical equation for the reaction of sulfur with
- O2(g).
- S2−(aq).
- F2(g).
- HNO3(aq).
Answer
-
-
-
- S8 + 8O2 8SO2(g)
- S8(s) + 8S2−(aq) → 8S22−(aq)
- S8(s) + 24F2(g) → 8SF6(g)
- S8(s) + 48HNO3(aq) → 8H2SO4(aq) + 48NO2(g) + 16H2O(l)




